Piperine Induces Apoptosis and Cell Cycle Arrest via Multiple Oxidative Stress Mechanisms and Regulation of PI3K/Akt and MAPK Signaling in Colorectal Cancer Cells
- PMID: 40722996
- PMCID: PMC12292380
- DOI: 10.3390/antiox14070892
Piperine Induces Apoptosis and Cell Cycle Arrest via Multiple Oxidative Stress Mechanisms and Regulation of PI3K/Akt and MAPK Signaling in Colorectal Cancer Cells
Abstract
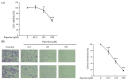
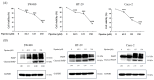
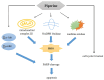
Piperine, a phytochemical alkaloid, exhibits notable anticancer properties in several cancer cell types. In this study, we investigated the mechanisms by which piperine induces cell death and apoptosis in colorectal cancer (CRC) cells, focusing on oxidative stress and key signaling pathways. Using MTT assay, flow cytometry, gene overexpression, and Western blot analysis, we observed that piperine significantly reduced cell viability, triggered G1 phase cell cycle arrest, and promoted apoptosis in DLD-1 cells. In addition, piperine effectively suppressed cell viability and induced apoptosis in other CRC cell lines, including SW480, HT-29, and Caco-2 cells. These effects were associated with increased intracellular reactive oxygen species (ROS) generation, mediated by the regulation of mitochondrial complex III, NADPH oxidase, and xanthine oxidase. Additionally, piperine modulated signaling pathways by inhibiting phosphoinositide 3-kinase (PI3K)/Akt, activating p38 and p-extracellular signal-regulated kinase (ERK). Pretreatment with antimycin A, apocynin, allopurinol, and PD98059, and the overexpression of p-Akt significantly recovered cell viability and reduced apoptosis, confirming the involvement of these pathways. This study is the first to demonstrate piperine induces apoptosis in CRC cells through a multifaceted oxidative stress mechanism and by critically modulating PI3K/Akt and ERK signaling pathways.
Keywords: MAPK; PI3K/Akt; apoptosis; cell cycle arrest; colorectal cancer; oxidative stress; piperine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

