Patient-Derived Gastric Cancer Assembloid Model Integrating Matched Tumor Organoids and Stromal Cell Subpopulations
- PMID: 40723172
- PMCID: PMC12293640
- DOI: 10.3390/cancers17142287
Patient-Derived Gastric Cancer Assembloid Model Integrating Matched Tumor Organoids and Stromal Cell Subpopulations
Abstract
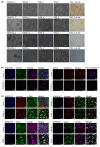
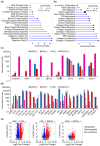
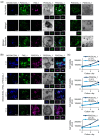
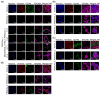
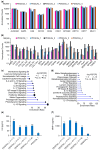
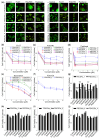
Background/Purpose: Conventional three-dimensional in vitro tumor models often fail to fully capture the complexity of the tumor microenvironment, particularly the diverse populations of cancer-associated fibroblasts that contribute to poor prognosis and treatment resistance. The purpose of this study is to develop a patient-specific gastric cancer assembloid model that integrates tumor epithelial cells with matched stromal cell subtypes, each derived using tailored growth media to enhance cancer preclinical research and advance personalized therapeutic strategies. Methods: Tumor tissue was dissociated, and cells expanded in media for organoids, mesenchymal stem cells, fibroblasts, or endothelial cells. The resulting tumor-derived subpopulations were co-cultured in an optimized assembloid medium supporting each cell type's growth. Biomarker expression was assessed by immunofluorescence staining, and transcriptomic profiles were analyzed by RNA sequencing. Drug responsiveness was evaluated using cell viability assays following treatment with various therapeutic agents. Results: The optimized co-culture conditions yielded assembloids that closely mimicked the cellular heterogeneity of primary tumors, confirmed by the expression of epithelial and stromal markers. Compared to monocultures, the assembloids showed higher expression of inflammatory cytokines, extracellular matrix remodeling factors, and tumor progression-related genes across different organoids and stromal ratios. Drug screening revealed patient- and drug-specific variability. While some drugs were effective in both organoid and assembloid models, others lost efficacy in the assembloids, highlighting the critical role of stromal components in modulating drug responses. Conclusions: This assembloid system offers a robust platform to study tumor-stroma interactions, identify resistance mechanisms, and accelerate drug discovery and personalized therapeutic strategies for gastric cancer.
Keywords: assembloids; drug resistance; gastric cancer; organoids; personalized medicine; stromal cell subpopulations; tumor microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources

