Toxicity Profiles of Antibody-Drug Conjugates: Synthesis and Graphical Insights to Optimize Patient-Centered Treatment Strategies for HER2-Negative Metastatic Breast Cancer
- PMID: 40723191
- PMCID: PMC12293329
- DOI: 10.3390/cancers17142307
Toxicity Profiles of Antibody-Drug Conjugates: Synthesis and Graphical Insights to Optimize Patient-Centered Treatment Strategies for HER2-Negative Metastatic Breast Cancer
Abstract
Background: The treatment options for HER2-negative metastatic breast cancer include targeted therapies, cytotoxic chemotherapies, and immunotherapy. However, limited specificity and inevitable resistance highlight the need for novel agents. Antibody-drug conjugates (ADCs), such as trastuzumab deruxtecan (T-DXd) and sacituzumab govitecan (SG), represent a breakthrough by selectively delivering cytotoxic agents to tumor cells, potentially improving the therapeutic index. Despite demonstrated efficacy, ADCs present toxicity profiles similar to conventional chemotherapy, alongside unique adverse events. In clinical practice, oncologists may face scenarios where both T-DXd and SG are treatment options in HER2-negative mBC. To enable shared decision-making, it is crucial to present a comprehensive overview that includes both efficacy data and detailed toxicity profiles. Our objective was to provide a pooled and informative synthesis of toxicities from pivotal studies, including graphical representations, to support informed, patient-centered medical decisions.
Methods: We reviewed safety data from phase 3 clinical trials in HER2-negative mBC: DESTINY-Breast04/DESTINY-Breast06 for T-DXd and ASCENT/TROPICS-02 for SG. Adverse event (AE) profiles, including frequency and severity, were extracted, and weighted means were calculated. Emerging ADCs such as datopotamab deruxtecan and patritumab deruxtecan were considered to contextualize future therapeutic decisions.
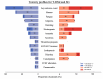
Results: Tables, bar plots and radar plots were generated. T-DXd demonstrated high rates of nausea (69.2%), fatigue (47.2%), and neutropenia (35.6%), with 52.7% experiencing grade ≥ 3 AEs. Notably, pneumonitis occurred in 10.7%, with grade ≥ 3 in 2.6%. SG showed a distinct AE profile, with higher incidences of neutropenia (67.1%), with grade ≥ 3 in 51.3%, and diarrhea (60.8%).
Conclusions: The choice between ADCs in HER2-negative metastatic BC when both T-DXd and SG are treatment options should consider toxicity profiles to optimize patient-centered treatment strategies. Tailoring ADC selection based on individual tolerance and preferences is critical for shared decision-making, and future research should focus on assessing the utility and acceptability of such clinical tools to guide treatment selection.
Keywords: antibody drug conjugate; breast cancer; safety; shared decision-making; toxicity.
Conflict of interest statement
A.d.N. declares the following competing interests: Gilead (lecture fees, congress invitations), Daiichi Sankyo (lecture fees, congress invitations), Seagen (consulting fees), Lilly (lecture fees, congress invitations, consulting fees), Novartis (consulting fees, congress invitations), MSD (congress invitations, lecture fees), Pfizer (research grants paid to institution, congress invitations). The other authors declare no conflicts of interest.
Figures
References
-
- Female Breast Cancer Subtypes—Cancer Stat Facts. [(accessed on 29 June 2025)]; Available online: https://seer.cancer.gov/statfacts/html/breast-subtypes.html.
-
- Gennari A., André F., Barrios C.H., Cortés J., de Azambuja E., DeMichele A., Dent R., Fenlon D., Gligorov J., Hurvitz S.A., et al. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer. Ann. Oncol. 2021;32:1475–1495. doi: 10.1016/j.annonc.2021.09.019. - DOI - PubMed
-
- Cortés J., Lipatov O., Im S.-A., Gonçalves A., Lee K.S., Schmid P., Tamura K., Testa L., Witzel I., Ohtani S., et al. LBA21—KEYNOTE-119: Phase III Study of Pembrolizumab (Pembro) versus Single-Agent Chemotherapy (Chemo) for Metastatic Triple Negative Breast Cancer (mTNBC) Ann. Oncol. 2019;30:v859–v860. doi: 10.1093/annonc/mdz394.010. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous