Animal Venoms as Potential Antitumor Agents Against Leukemia and Lymphoma
- PMID: 40723215
- PMCID: PMC12294047
- DOI: 10.3390/cancers17142331
Animal Venoms as Potential Antitumor Agents Against Leukemia and Lymphoma
Abstract
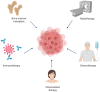
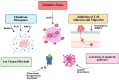
Leukemias and lymphomas are hematologic malignancies characterized by complex pathophysiological mechanisms and increasing global incidence. Despite advances in chemotherapy, immunotherapy, and targeted therapies, challenges such as drug resistance and relapse persist, necessitating novel therapeutic strategies. This review explores the cytotoxic potential of venoms derived from snakes, bees, and scorpions against leukemia and lymphoma cells. Numerous venom-derived components, such as L-amino acid oxidases (LAAOs), phospholipases A2 (PLA2s), and peptides like melittin, demonstrate selective antitumor activity through mechanisms involving oxidative stress, apoptosis induction, cell cycle arrest, and immunomodulation. These molecules exert their effects via mitochondrial pathways, caspase activation, and inhibition of pro-survival signaling cascades such as NF-κB and PI3K/Akt. Despite promising preclinical results, the clinical translation of these bioactive compounds remains limited due to challenges in standardization, delivery, and safety profiling. This review highlights recent advances in venom research, summarizes key molecular targets, and discusses future directions to harness venom-derived molecules as innovative therapies for hematological cancers.
Keywords: L-amino acid oxidase (LAAO); anticancer therapy; apoptosis; cytotoxicity; hematological malignancies; melittin; phospholipase A2 (PLA2).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Anti-Inflammatory Potential of Fish-Derived Bioactive Peptides: Molecular Mechanisms, Delivery Strategies, and Clinical Perspectives.Compr Rev Food Sci Food Saf. 2025 Jul;24(4):e70234. doi: 10.1111/1541-4337.70234. Compr Rev Food Sci Food Saf. 2025. PMID: 40679268 Review.
-
Deciphering the Neuroprotective Action of Bee Venom Peptide Melittin: Insights into Mechanistic Interplay.Mol Neurobiol. 2025 Jul;62(7):8738-8751. doi: 10.1007/s12035-025-04808-6. Epub 2025 Mar 4. Mol Neurobiol. 2025. PMID: 40038194 Review.
-
Phospholipase A2-A Significant Bio-Active Molecule in Honeybee (Apis mellifera L.) Venom.Molecules. 2025 Jun 17;30(12):2623. doi: 10.3390/molecules30122623. Molecules. 2025. PMID: 40572586 Free PMC article. Review.
-
Unraveling the mechanisms of antitumor action of Sophora flavescens flavonoids via network pharmacology and molecular simulation.In Silico Pharmacol. 2025 Mar 20;13(1):48. doi: 10.1007/s40203-025-00338-0. eCollection 2025. In Silico Pharmacol. 2025. PMID: 40125411
-
The therapeutic potential of bee venom-derived Apamin and Melittin conjugates in cancer treatment: A systematic review.Pharmacol Res. 2024 Nov;209:107430. doi: 10.1016/j.phrs.2024.107430. Epub 2024 Sep 26. Pharmacol Res. 2024. PMID: 39332751
References
Publication types
LinkOut - more resources
Full Text Sources

