Novel Therapeutics and the Path Toward Effective Immunotherapy in Malignant Peripheral Nerve Sheath Tumors
- PMID: 40723291
- PMCID: PMC12294001
- DOI: 10.3390/cancers17142410
Novel Therapeutics and the Path Toward Effective Immunotherapy in Malignant Peripheral Nerve Sheath Tumors
Abstract
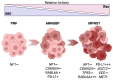
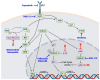
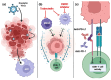
Malignant Peripheral Nerve Sheath Tumors (MPNSTs) are a deadly subtype of soft tissue sarcoma for which effective therapeutic options are lacking. Currently, the best treatment for MPNSTs is complete surgical resection with wide negative margins, but this is often complicated by the tumor size and location and/or the presence of metastases. Radiation or chemotherapy may be combined with surgery, but patient responses are poor. Targeted treatments, including small-molecule inhibitors of oncogenic proteins such as mitogen-activated protein kinase kinase (MEK), cyclin-dependent kinases 4 and 6 (CDK4/6), and Src-homology 2 domain-containing phosphatase 2 (SHP2), are promising therapeutics for MPNSTs, especially when combined together, but they have yet to gain approval. Immunotherapeutic approaches have been revolutionary for the treatment of some other cancers, but their utility as single agents in sarcoma is limited and not approved for MPNSTs. The immunosuppressive niche of MPNSTs is thought to confer inherent treatment resistance, particularly to immunotherapies. Remodeling an inherently "cold" tumor microenvironment into a "hot" immune milieu to bolster the anti-tumor activity of immunotherapies is of great interest throughout the cancer community. This review focuses on novel therapeutics that target dysregulated factors and pathways in MPNSTs, as well as different types of immunotherapies currently under investigation for this disease. We also consider how certain therapeutics may be combined to remodel the MPNST immune microenvironment and thereby generate a durable anti-tumor immune response to immunotherapy.
Keywords: MPNST; immunotherapy; targeted therapy.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures
Similar articles
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
References
-
- Martin E., Lamba N., Flucke U.E., Verhoef C., Coert J.H., Versleijen-Jonkers Y.M.H., Desar I.M.E. Non-cytotoxic systemic treatment in malignant peripheral nerve sheath tumors (MPNST): A systematic review from bench to bedside. Crit. Rev. Oncol. Hematol. 2019;138:223–232. doi: 10.1016/j.critrevonc.2019.04.007. - DOI - PubMed
-
- Zehou O., Fabre E., Zelek L., Sbidian E., Ortonne N., Banu E., Wolkenstein P., Valeyrie-Allanore L. Chemotherapy for the treatment of malignant peripheral nerve sheath tumors in neurofibromatosis 1: A 10-year institutional review. Orphanet J. Rare Dis. 2013;8:127. doi: 10.1186/1750-1172-8-127. - DOI - PMC - PubMed
-
- Watson K.L., Al Sannaa G.A., Kivlin C.M., Ingram D.R., Landers S.M., Roland C.L., Cormier J.N., Hunt K.K., Feig B.W., Ashleigh Guadagnolo B., et al. Patterns of recurrence and survival in sporadic, neurofibromatosis Type 1-associated, and radiation-associated malignant peripheral nerve sheath tumors. J. Neurosurg. 2017;126:319–329. doi: 10.3171/2015.12.JNS152443. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

