Design of a Multi-Epitope Vaccine Candidate Against Infectious Laryngotracheitis Virus Affecting Poultry by Computational Approaches
- PMID: 40723326
- PMCID: PMC12292712
- DOI: 10.3390/biology14070765
Design of a Multi-Epitope Vaccine Candidate Against Infectious Laryngotracheitis Virus Affecting Poultry by Computational Approaches
Abstract
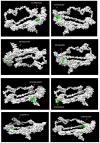
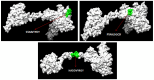
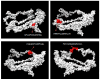
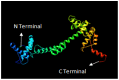
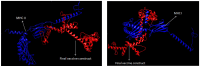
Infectious laryngotracheitis (ILT) is a severe upper respiratory disease highly contagious in chickens that causes a huge economic impact on the poultry industry all over the world. The current study aimed to design a multi-epitope-based vaccine candidate using envelope glycoprotein B and glycoprotein D of the ILT virus using an immune informatics approach. The glycoproteins B and D are crucial for attachment as well as entry of ILT virus inside the cell, which makes them a potential option for designing vaccine candidates. The prediction of epitopes, viz. helper T lymphocyte, cytotoxic T lymphocyte and interferon-gamma producing epitopes, was performed and high-scoring predicted epitopes were joined in an organized manner using suitable linkers to design the final vaccine candidate. The avian beta-defensin 1 was included as an adjuvant in the amino-terminal of the vaccine design that possesses antimicrobial activity and histidine residues at the carboxy-terminal for the purpose of purification. The final vaccine candidate was evaluated for its physicochemical characteristics, solubility, antigenicity, stability, and allergenicity and validated for its modeling. Molecular docking, binding affinity, and interacting residues between the vaccine candidate and immune receptors, viz. TLR 3, MHC Class I and Class II were assessed. Further, to assess the immune response profile generated by the final vaccine design, an insilico immune simulation study was also performed. The findings of this study revealed that the final vaccine candidate was antigenic, nonallergenic, stable, interacted with immune receptors, and able to produce antibodies as well as cellular immune responses against ILTV infection.
Keywords: avian beta-defensin 1; glycoprotein B; glycoprotein D; immunoinformatics; infectious laryngotracheitis virus; multi-epitope vaccine.
Conflict of interest statement
The authors declare that there are no conflicts of interest in this work.
Figures










Similar articles
-
Design of a multi-epitope Zika virus vaccine candidate - an in-silico study.J Biomol Struct Dyn. 2023 Jun;41(9):3762-3771. doi: 10.1080/07391102.2022.2055648. Epub 2022 Mar 23. J Biomol Struct Dyn. 2023. PMID: 35318896
-
Design and Validation of a Multi-Epitope mRNA Vaccine Construct Against Human Monkeypox Virus (hMPXV) by Annotating Protein of Intracellular Mature Virus (IMV) Form of hMPXV.Biomedicines. 2025 Jun 11;13(6):1439. doi: 10.3390/biomedicines13061439. Biomedicines. 2025. PMID: 40564159 Free PMC article.
-
Towards precision epitopes based vaccine against Enterococcus faecalis by integrating vaccinomics, reverse vaccinology and biophysics approaches.Biochem Biophys Rep. 2025 Jun 10;43:102082. doi: 10.1016/j.bbrep.2025.102082. eCollection 2025 Sep. Biochem Biophys Rep. 2025. PMID: 40546343 Free PMC article.
-
Immunoinformatics-Driven Design of a Multi-Epitope Vaccine Targeting Simian Virus VP1 Major Capsid Protein for Oncogenic Viral Infection Prevention.Rev Med Virol. 2025 Sep;35(5):e70065. doi: 10.1002/rmv.70065. Rev Med Virol. 2025. PMID: 40781525 Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- García M., Spatz S.J., Guy J.S. Infectious laryngotracheitis. In: Swayne D.E., Glisson J.R., McDougald L.R., Nolan L.K., Suarez D.L., Nair V., editors. Diseases of Poultry. 13th ed. Blackwell Publishing; Ames, IA, USA: 2013. pp. 161–179.
LinkOut - more resources
Full Text Sources
Research Materials

