Colonic Aging and Colorectal Cancer: An Unignorable Interplay and Its Translational Implications
- PMID: 40723364
- PMCID: PMC12292539
- DOI: 10.3390/biology14070805
Colonic Aging and Colorectal Cancer: An Unignorable Interplay and Its Translational Implications
Abstract
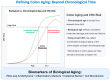
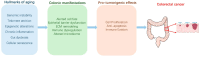
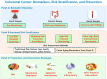
Colorectal cancer (CRC) incidence increases markedly with age, yet chronological age is an inadequate proxy for the complex biological processes involved. Colon aging, the intrinsic biological aging of the colonic tissue, is emerging as a crucial, active driver of CRC development. This review comprehensively analyzes the interplay between colon aging and CRC pathogenesis by examining fundamental hallmarks of aging-such as altered tissue homeostasis, epigenetic dysregulation, and microenvironmental shifts including chronic inflammation (inflammaging), gut microbiome dysbiosis, and extracellular matrix remodeling-manifest specifically within the aging colon to synergistically foster a pro-tumorigenic environment. Key findings synthesized from the literature highlight the critical roles of impaired colonic stem cell function, epithelial barrier disruption ("leaky gut"), persistent low-grade inflammation, and altered microbial communities and their metabolites in promoting CRC initiation and progression. Translating this mechanistic understanding holds significant promise: insights from colon aging research can inform novel biomarkers for improved early detection and risk stratification, guide the development of personalized preventative strategies and therapeutic interventions targeting aging pathways, and underpin public health initiatives focused on healthy colon aging. Ultimately, recognizing colon aging as a modifiable contributor to CRC offers a powerful avenue to potentially reduce CRC incidence and enhance patient outcomes, particularly in the vulnerable aging population.
Keywords: biological aging; colon aging; colorectal cancer; dysbiosis; early detection; inflammation; microenvironment; prevention; senescence; translational research.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
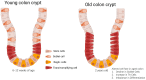

Similar articles
-
High-throughput N-glycan analysis in aging and inflammaging: State of the art and future directions.Semin Immunol. 2024 May;73:101890. doi: 10.1016/j.smim.2024.101890. Epub 2024 Oct 9. Semin Immunol. 2024. PMID: 39383621 Review.
-
Cracking the Code of Colorectal Cancer Screening: An Overview With a Focus on Current and Emerging Screening Methods.Cureus. 2025 May 24;17(5):e84724. doi: 10.7759/cureus.84724. eCollection 2025 May. Cureus. 2025. PMID: 40551903 Free PMC article. Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
References
-
- Mittal P., Battaglin F., Yang Y., Soni S., Stintzing S., Parikh A.R., Ashouri K., Algaze S., Jayachandran P., Torres-Gonzalez L. Genetic Polymorphisms in MHC Classes I and II Predict Outcomes in Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2025;26:2556. doi: 10.3390/ijms26062556. - DOI - PMC - PubMed
-
- Froelich W. Accelerated Aging Increases Risk of Early-Onset Cancers. Oncol. Times. 2024;46:27–33. doi: 10.1097/01.COT.0001026252.73088.6b. - DOI
Publication types
Grants and funding
- 2023YFC3603300/National Key Research and Development Project of China
- 82273166/National Natural Science Foundation of China
- 202201AY070001-266/major joint project of Yunnan Provincial Department of Science and Technology and Kunming Medical University on applied basic research
- YDYXJJ2024-0008/Yunnan University Medical Research Foundation
- 202401BF070001-017/Yunnan Provincial Department of Science and Technology - Yunnan University Joint Special Program for the Double First-Class Initiative
LinkOut - more resources
Full Text Sources

