Functions of Intrinsically Disordered Regions
- PMID: 40723369
- PMCID: PMC12292460
- DOI: 10.3390/biology14070810
Functions of Intrinsically Disordered Regions
Abstract
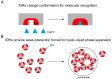
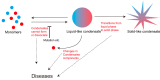
Intrinsically disordered regions (IDRs), defined as protein segments lacking stable tertiary structures, are ubiquitously present in the human proteome and enriched with disease-associated mutations. IDRs harbor molecular recognition features (MoRFs) and post-translational modification sites (e.g., phosphorylation), enabling dynamic intermolecular interactions through conformational plasticity. Furthermore, IDRs drive liquid-liquid phase separation (LLPS) of biomacromolecules via multivalent interactions such as electrostatic attraction and pi-pi interactions, generating biomolecular condensates that are essential throughout the cellular lifecycle. These condensates separate intracellular space, forming a physical barrier to avoid interference between other molecules, thereby improving reaction specificity and efficiency. As a dynamically equilibrated process, LLPS formation and maintenance are regulated by multiple factors, endowing the condensates with rapid responsiveness to environmental cues and functional versatility in modulating diverse signaling cascades. Consequently, disruption of LLPS homeostasis can derail its associated biological processes, ultimately contributing to disease pathogenesis. Moreover, precisely because liquid-liquid phase separation (LLPS) is co-regulated by multiple factors, it may provide novel insights into the pathogenic mechanisms of disorders such as autism spectrum disorder (ASD), which result from the cumulative effects of multiple etiological factors.
Keywords: intrinsically disordered regions; liquid–liquid phase separation; molecular recognition features; post-translation modification.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Nonspecific interactions can lead to liquid-liquid phase separation in coiled-coil proteins models.bioRxiv [Preprint]. 2025 May 15:2025.05.09.653163. doi: 10.1101/2025.05.09.653163. bioRxiv. 2025. PMID: 40463193 Free PMC article. Preprint.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Structural dynamics of IDR interactions in human SFPQ and implications for liquid-liquid phase separation.Acta Crystallogr D Struct Biol. 2025 Jul 1;81(Pt 7):357-379. doi: 10.1107/S2059798325005303. Epub 2025 Jun 27. Acta Crystallogr D Struct Biol. 2025. PMID: 40574713 Free PMC article.
-
Small Molecules as Regulators of Liquid-Liquid Phase Separation: Mechanisms and Strategies for New Drug Discovery.FASEB J. 2025 Jul 15;39(13):e70773. doi: 10.1096/fj.202501476R. FASEB J. 2025. PMID: 40616404 Review.
-
Interplay between posttranslational modifications and liquid‒liquid phase separation in tumors.Cancer Lett. 2024 Mar 1;584:216614. doi: 10.1016/j.canlet.2024.216614. Epub 2024 Jan 19. Cancer Lett. 2024. PMID: 38246226 Review.
References
-
- Williams R.M., Obradovi Z., Mathura V., Braun W., Garner E.C., Young J., Takayama S., Brown C.J., Dunker A.K. The protein non-folding problem: Amino acid determinants of intrinsic order and disorder; Proceedings of the Pacific Symposium on Biocomputing. Pacific Symposium on Biocomputing; Waimea, HI, USA. 3–7 January 2001; pp. 89–100. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous