The Proteomic Landscape of Parkin-Deficient and Parkin-Overexpressing Rat Nucleus Accumbens: An Insight into the Role of Parkin in Methamphetamine Use Disorder
- PMID: 40723830
- PMCID: PMC12292523
- DOI: 10.3390/biom15070958
The Proteomic Landscape of Parkin-Deficient and Parkin-Overexpressing Rat Nucleus Accumbens: An Insight into the Role of Parkin in Methamphetamine Use Disorder
Abstract
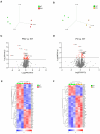
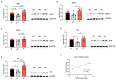
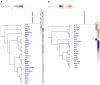
In recent years, methamphetamine (METH) misuse in the US has been rapidly increasing, and there is no FDA-approved pharmacotherapy for METH use disorder (MUD). We previously determined that ubiquitin-protein ligase parkin is involved in the regulation of METH addictive behaviors in rat models of MUD. Parkin is not yet a "druggable" drug target; therefore, this study aimed to determine which biological processes, pathways, and proteins downstream of parkin are likely drug targets against MUD. Employing young adult Long Evans male rats with parkin deficit or excess in the nucleus accumbens (NAc), label-free proteomics, and molecular biology, we determined that the pathways downstream of parkin that are candidates for regulating METH addictive behaviors in young adult male rats are mitochondrial respiration, oxidative stress, AMPA receptor trafficking, GABAergic neurotransmission, and actin cytoskeleton dynamics.
Keywords: methamphetamine; mitochondria; nucleus accumbens; parkin; proteome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- NIDA . Overdose Death Rates. NIDA; North Bethesda, MD, USA: 2023.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

