Therapeutic Plasma Exchange: Current and Emerging Applications to Mitigate Cellular Signaling in Disease
- PMID: 40723872
- PMCID: PMC12292254
- DOI: 10.3390/biom15071000
Therapeutic Plasma Exchange: Current and Emerging Applications to Mitigate Cellular Signaling in Disease
Abstract
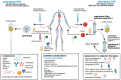
Therapeutic plasma exchange (TPE) is a blood purification technique which functions to remove pathological plasma constituents such as autoantibodies, inflammatory cytokines, immune complexes, and extracellular vesicles (EVs) that contribute to a range of disease states. In this review, we examine current and emerging indications for TPE across cardiovascular, metabolic, neurological, inflammatory, and oncological diseases. We cover emerging preclinical animal models and new applications, emphasizing the roles of cellular signaling and EV biology in mediating plasma functions, and discuss unique therapeutic "windows of opportunity" offered by TPE. We conclude that TPE is underutilized in both preventative and precision medicine, and that next generation TPE therapies will involve personalized plasma biomarker and modulation feedback, with synergistic plasma infusion therapies to mitigate age associated disease and promote tissue rejuvenation.
Keywords: apheresis; blood exchange; cancer; diabetes; extracellular vesicles; inflammation; metabolic disease; neurological disease; plasmapheresis; therapeutic plasma exchange.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Mathew J., Sankar P., Varacallo M.A. Physiology, Blood Plasma. Statpearls; Treasure Island, FL, USA: 2025. - PubMed
-
- Abel J.J., Rowntree L.G., Turner B.B. On the Removal of Diffusible Substances from the Circulating Blood of Living Animals by Dialysis Ii. Some Constituents of the Blood. J. Pharmacol. Exp. Ther. 1914;5:611–623. doi: 10.1016/S0022-3565(25)08254-0. - DOI
-
- Rivera A.M., Strauss K.W., Van Zundert A., Mortier E. The History of Peripheral Intravenous Catheters: How Little Plastic Tubes Revolutionized Medicine. Acta Anaesthesiol. Belg. 2005;56:271–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

