A Novel Microencapsulated Bovine Recombinant Interferon Tau Formulation for Luteolysis Modulation in Cattle
- PMID: 40723881
- PMCID: PMC12293041
- DOI: 10.3390/biom15071009
A Novel Microencapsulated Bovine Recombinant Interferon Tau Formulation for Luteolysis Modulation in Cattle
Abstract
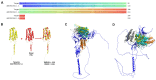
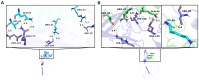
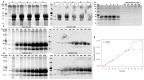
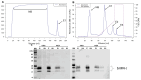
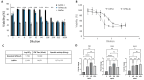
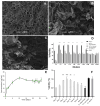
Early embryonic loss is a major cause of reproductive inefficiency in cattle, primarily due to premature luteolysis. Interferon tau (IFN-τ), secreted by the trophoblast, plays a critical role in maternal recognition of pregnancy by maintaining corpus luteum function. However, its practical application has been limited by its rapid degradation and short half-life in vivo. Here, we developed a novel formulation of recombinant bovine IFN-τ, combining chitosan-based microencapsulation with starch-chitosan hydrogel delivery, enabling sustained intrauterine release. This dual-delivery strategy offers a significant improvement over conventional IFN-τ administration methods that rely on repeated intrauterine infusions of soluble protein. The rbIFN-τ was expressed in Pichia pastoris, purified to 90.1% homogeneity, and structurally validated via homology modeling and molecular docking, confirming its interaction with type I interferon receptors. The encapsulated formulation retained antiviral activity, stimulated transcription of interferon-stimulated genes (PKR, OAS1, OAS2), and showed sustained release in vitro for up to 26 days. In vivo evaluation demonstrated safety and biological efficacy, with treated cattle showing inhibited luteolysis, sustained serum progesterone levels, and preserved corpus luteum integrity. This formulation represents a promising biotechnological approach to improve reproductive efficiency through a long-acting, species-specific IFN-τ delivery system.
Keywords: interferon tau; luteolysis modulation; microencapsulation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Delayed luteolysis and suppression of testosterone secretion after recombinant ovine interferon treatment in goats (Capra hircus).J Reprod Fertil. 1994 Nov;102(2):301-4. doi: 10.1530/jrf.0.1020301. J Reprod Fertil. 1994. PMID: 7861381
-
Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes.Cochrane Database Syst Rev. 2015 Mar 23;2015(3):CD007754. doi: 10.1002/14651858.CD007754.pub3. Cochrane Database Syst Rev. 2015. PMID: 25803792 Free PMC article.
-
Aminoadamantanes for chronic hepatitis C.Cochrane Database Syst Rev. 2014 May 3;2014(5):CD010125. doi: 10.1002/14651858.CD010125.pub2. Cochrane Database Syst Rev. 2014. PMID: 24793264 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
References
-
- Williams G.W., Anderson D.P. Choices Magazine 1 The Latin American Livestock Industry: Growth and Challenges. 2020. [(accessed on 1 January 2020)]. Available online: http://www.choicesmagazine.org/choices-magazine/submitted-articles/the-l....
-
- Gädicke L’Huissier P., Chihuailaf R., Letelier R., Allende R., Ruiz A., Junod T., Gädicke L’Huissier P., Chihuailaf R., Letelier R., Allende R., et al. Monitoring Different Causal Patterns of Bovine Abortion Syndrome. Vet. México OA. 2022;9:e652. doi: 10.22201/fmvz.24486760e.2022.652. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

