Galectin-3: Integrator of Signaling via Hexosamine Flux
- PMID: 40723900
- PMCID: PMC12293996
- DOI: 10.3390/biom15071028
Galectin-3: Integrator of Signaling via Hexosamine Flux
Abstract
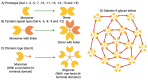
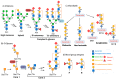
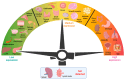
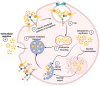
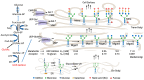
Galectin-3 (Gal-3) is a β-galactoside-binding lectin that mediates diverse signaling events in multiple cell types, including immune cells. It is also a prognostic indicator for multiple clinically important disorders, including cardiovascular disease. Gal-3 binds to cell surface glycans to form lattices that modulate surface receptor signaling and internalization. However, the tissue-specific regulation of Gal-3 surface expression remains poorly understood. Here, we review evidence for the involvement of Gal-3 in cell surface signaling, intranuclear events, and intracellular trafficking. Our focus will be on the O-GlcNAc modification as a regulator of Gal-3 biosynthesis, non-canonical secretion, and recycling. We argue that the nutrient-driven cytoplasmic hexosamine biosynthetic pathway (HBP) and endomembrane transport mechanisms generate unique pools of nucleotide sugars. The differing levels of nucleotide sugars in the cytosol, endoplasmic reticulum (ER), and Golgi apparatus generate differential thresholds for the responsiveness of O-GlcNAc cycling, N- and O-linked glycan synthesis/branching, and glycolipid synthesis. By regulating Gal-3 synthesis and non-canonical secretion, O-GlcNAc cycling may serve as a nexus constraining Gal-3 cell surface expression and lattice formation. This homeostatic feedback mechanism would be critical under conditions where extensive glycan synthesis and branching in the endomembrane system and on the cell surface are maintained by elevated hexosamine synthesis. Thus, O-GlcNAc cycling and Gal-3 synergize to regulate Gal-3 secretion and influence cellular signaling. In humans, Gal-3 serves as an early-stage prognostic indicator for heart disease, kidney disease, viral infection, autoimmune disease, and neurodegenerative disorders. Since O-GlcNAc cycling has also been linked to these pathologic states, exploring the interconnections between O-GlcNAc cycling and Gal-3 expression and synthesis is likely to emerge as an exciting area of research.
Keywords: Galectin-3; HBP; N-Glycan; O-GlcNAcylation; UDP-GlcNAc.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Restriction of Human Cytomegalovirus Infection by Galectin-9.J Virol. 2019 Jan 17;93(3):e01746-18. doi: 10.1128/JVI.01746-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30487283 Free PMC article.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

