Electrophysiological Characterization of Sex-Dependent Hypnosis by an Endogenous Neuroactive Steroid Epipregnanolone
- PMID: 40723904
- PMCID: PMC12293945
- DOI: 10.3390/biom15071033
Electrophysiological Characterization of Sex-Dependent Hypnosis by an Endogenous Neuroactive Steroid Epipregnanolone
Abstract
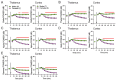
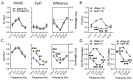
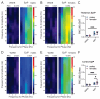
Neuroactive steroids (NAS) have long been recognized for their hypnotic and anesthetic properties in both clinical and preclinical settings. While sex differences in NAS sensitivity are acknowledged, the underlying mechanisms remain poorly understood. Here, we examined sex-specific responses to an endogenous NAS epipregnanolone (EpiP) in wild-type mice using behavioral assessment of hypnosis (loss of righting reflex, LORR) and in vivo electrophysiological recordings. Specifically, local field potentials (LFPs) were recorded from the central medial thalamus (CMT) and electroencephalogram (EEG) signals were recorded from the barrel cortex. We found that EpiP-induced LORR exhibited clear sex differences, with females showing increased sensitivity. Spectral power analysis and thalamocortical (TC) and corticocortical (CC) phase synchronization further supported enhanced hypnotic susceptibility in female mice. Our findings reveal characteristic sex-dependent effects of EpiP on the synchronized electrical activity in both thalamus and cortex. These results support renewed exploration of endogenous NAS as clinically relevant anesthetic agents.
Keywords: electroencephalogram; general anesthetics; hypnosis; neuroactive steroids; sex differences.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Comparative electrophysiological study of neuroactive steroid-induced hypnosis in mice: sex and drug-specific differences.Exp Biol Med (Maywood). 2025 Jun 10;250:10550. doi: 10.3389/ebm.2025.10550. eCollection 2025. Exp Biol Med (Maywood). 2025. PMID: 40557248 Free PMC article.
-
Different roles of T-type calcium channel isoforms in hypnosis induced by an endogenous neurosteroid epipregnanolone.Neuropharmacology. 2021 Oct 1;197:108739. doi: 10.1016/j.neuropharm.2021.108739. Epub 2021 Jul 31. Neuropharmacology. 2021. PMID: 34339750 Free PMC article.
-
In vivo silencing of the thalamic CaV3.1 voltage-gated calcium channels demonstrates their region-specific role in anesthetic mediated hypnosis.Exp Biol Med (Maywood). 2025 May 16;250:10553. doi: 10.3389/ebm.2025.10553. eCollection 2025. Exp Biol Med (Maywood). 2025. PMID: 40453358 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Coulter I., Timic Stamenic T., Eggan P., Fine B.R., Corrigan T., Covey D.F., Yang L., Pan J.Q., Todorovic S.M. Different Roles of T-Type Calcium Channel Isoforms in Hypnosis Induced by an Endogenous Neurosteroid Epipregnanolone. Neuropharmacology. 2021;197:108739. doi: 10.1016/j.neuropharm.2021.108739. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

