Co-Inhibition of PARP and STAT3 as a Promising Approach for Triple-Negative Breast Cancer
- PMID: 40723906
- PMCID: PMC12292728
- DOI: 10.3390/biom15071035
Co-Inhibition of PARP and STAT3 as a Promising Approach for Triple-Negative Breast Cancer
Abstract
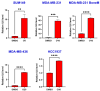
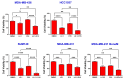
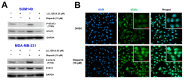
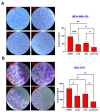
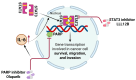
Triple-negative breast cancer (TNBC) is a highly aggressive subtype known for its rapid metastatic potential. Despite its severity, treatment options for TNBC remain limited. Olaparib, an FDA-approved PARP inhibitor, has been used to treat germline BRCA-mutated TNBC in both metastatic and high-risk early-stage settings. However, acquired resistance to PARP inhibitors and their limited applicability in non-BRCA TNBCs are now two major growing clinical problems. Activation of the IL-6/STAT3 signaling cascade has been implicated in therapeutic resistance. In this study, we evaluated the combined effects of the PARP inhibitor olaparib and the STAT3 inhibitor LLL12B in human TNBC cell lines with both BRCA mutations and wild-type BRCA status. Our results demonstrate that the PARP inhibitor olaparib can induce increased interleukin-6 (IL-6) in TNBC cells, with ELISA showing a 2- to 39-fold increase across five cell lines. MTT assays revealed that knocking down or inhibiting STAT3, a key downstream effector of the IL-6/GP130 pathway, sensitizes TNBC cells to olaparib. Treatment with either olaparib or LLL12B alone reduced TNBC cell viability, migration, and invasion. Notably, their combined administration produced a markedly enhanced inhibitory effect compared to individual treatments, regardless of BRCA mutation status. These findings highlight the potential of dual PARP and STAT3 inhibition as a novel targeted therapeutic strategy for both BRCA-mutant and BRCA-proficient TNBC.
Keywords: BRCA; IL-6/STAT3 signaling; PARP inhibitor; STAT3 inhibition; triple-negative breast cancer (TNBC).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- del Rivero J., Kohn E.C. PARP Inhibitors: The Cornerstone of DNA Repair-Targeted Therapies. Oncology. 2017;31:265–273. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

