Effect of Pefloxacin on Clostridioides difficile R20291 Persister Cells Formation
- PMID: 40723930
- PMCID: PMC12291809
- DOI: 10.3390/antibiotics14070628
Effect of Pefloxacin on Clostridioides difficile R20291 Persister Cells Formation
Abstract
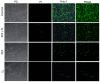
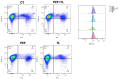
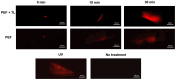
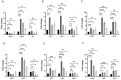
Clostridioides difficile is a Gram-positive bacterium recognized for its ability to produce toxins and form spores. It is mainly accountable for the majority of instances of antibiotic-related diarrhea. Background. Bacterial persister represent a minor fraction of the population that shows temporary tolerance to bactericidal agents, and they pose considerable medical issues because of their link to the rise of antibiotic resistance and challenging chronic or recurrent infections. Our previous research has shown a persister-like phenotype associated with treatments that include pefloxacin. Nonetheless, the mechanism is still mostly unclear, mainly because of the difficulty in isolating this small group of cells. Objectives. To enhance the understanding of C. difficile persister cells, we made an enrichment and characterization of these cells from bacterial cultures during the exponential phase under pefloxacin treatment and lysis treatment. Results. We demonstrate the appearance of cells with lower metabolism and DNA damage. Furthermore, we noted the participation of toxin-antitoxin systems and Clp proteases in the generation of persister cells. Conclusions. This work demonstrates the formation of C. difficile persister cells triggered by a lethal concentration of pefloxacin.
Keywords: Clostridioides difficile; pefloxacin; persister cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
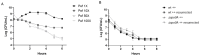
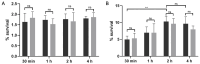
Similar articles
-
Antibiotic treatment for Clostridium difficile-associated diarrhea in adults.Cochrane Database Syst Rev. 2005 Jan 25;(1):CD004610. doi: 10.1002/14651858.CD004610.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2007 Jul 18;(3):CD004610. doi: 10.1002/14651858.CD004610.pub3. PMID: 15674956 Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults.Cochrane Database Syst Rev. 2017 Mar 3;3(3):CD004610. doi: 10.1002/14651858.CD004610.pub5. Cochrane Database Syst Rev. 2017. PMID: 28257555 Free PMC article.
-
Fecal microbiota transplantation for the treatment of recurrent Clostridioides difficile (Clostridium difficile).Cochrane Database Syst Rev. 2023 Apr 25;4(4):CD013871. doi: 10.1002/14651858.CD013871.pub2. Cochrane Database Syst Rev. 2023. PMID: 37096495 Free PMC article.
-
Characterization of Flagellum and Toxin Phase Variation in Clostridioides difficile Ribotype 012 Isolates.J Bacteriol. 2018 Jun 25;200(14):e00056-18. doi: 10.1128/JB.00056-18. Print 2018 Jul 15. J Bacteriol. 2018. PMID: 29735765 Free PMC article.
References
-
- Di Bella S., Sanson G., Monticelli J., Zerbato V., Principe L., Giuffrè M., Luzzati R. Clostridioides difficile infection: History, epidemiology, risk factors, prevention, clinical manifestations, treatment, and future options. Clin. Microbiol. Rev. 2024;37:e0013523. doi: 10.1128/cmr.00135-23. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

