Dual Redox Targeting by Pyrroloformamide A and Silver Ions Enhances Antibacterial and Anti-Biofilm Activity Against Carbapenem-Resistant Klebsiella pneumoniae
- PMID: 40723943
- PMCID: PMC12291900
- DOI: 10.3390/antibiotics14070640
Dual Redox Targeting by Pyrroloformamide A and Silver Ions Enhances Antibacterial and Anti-Biofilm Activity Against Carbapenem-Resistant Klebsiella pneumoniae
Abstract
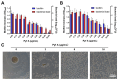
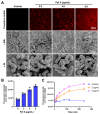
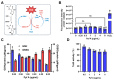
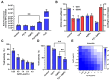
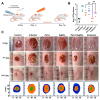
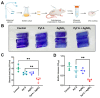
Background: Dithiolopyrrolones (DTPs), such as holomycin and thiolutin, exhibit potent antibacterial activities. DTPs contain a disulfide within a unique bicyclic scaffold, which may chelate metal ions and disrupt metal-dependent cellular processes once the disulfide is reductively transformed to thiols. However, the contribution of the intrinsic redox mechanism of DTPs to their antibacterial activity remains unclear. Herein we used pyrroloformamide (Pyf) A, a DTP with a unique formyl substituent, as a prototype to study the antibacterial potential and mechanism against ESKAPE pathogens, in particular carbapenem-resistant Klebsiella pneumoniae (CRKP). Methods: The antibacterial and anti-biofilm activities of Pyf A were mainly assessed against clinical CRKP isolates. Propidium iodide staining, scanning electron microscopy, glutathione (GSH) quantification, and reactive oxygen species (ROS) analysis were utilized to infer its anti-CRKP mechanism. The synergistic antibacterial effects of Pyf A and AgNO3 were evaluated through checkerboard and time-kill assays, as well as in vivo murine wound and catheter biofilm infection models. Results: Pyf A exhibited broad-spectrum antibacterial activity against ESKAPE pathogens with minimum inhibitory concentrations ranging from 0.25 to 4 μg/mL. It also showed potent anti-biofilm effects against CRKP. Pyf A disrupted the cell membranes of CRKP and markedly depleted intracellular GSH without triggering ROS accumulation. Pyf A and AgNO3 showed synergistic anti-CRKP activities in vitro and in vivo, by disrupting both GSH- and thioredoxin-mediated redox homeostasis. Conclusions: Pyf A acts as a GSH-depleting agent and, when combined with AgNO3, achieves dual-targeted disruption of bacterial thiol redox systems. This dual-targeting strategy enhances antibacterial efficacy of Pyf A and represents a promising therapeutic approach to combat CRKP infections.
Keywords: AgNO3; carbapenem-resistant Klebsiella pneumoniae; glutathione; pyrroloformamide A; redox homeostasis; thioredoxin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. 2024. [(accessed on 17 May 2025)]. Available online: https://www.who.int/publications/i/item/9789240093461. - PubMed
-
- Antimicrobial Resistance, Hypervirulent Klebsiella pneumoniae—Global Situation. 2024. [(accessed on 17 May 2025)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON527.
Grants and funding
LinkOut - more resources
Full Text Sources

