Evaluating the Feasibility and Acceptability of a Prototype Hospital Digital Antibiotic Review Tracking Toolkit: A Qualitative Study Using the RE-AIM Framework
- PMID: 40723963
- PMCID: PMC12291640
- DOI: 10.3390/antibiotics14070660
Evaluating the Feasibility and Acceptability of a Prototype Hospital Digital Antibiotic Review Tracking Toolkit: A Qualitative Study Using the RE-AIM Framework
Abstract
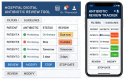
Background: Internationally, digital health interventions have increasingly been adopted within hospital settings. Optimising their clinical implementation requires user involvement, but there is a lack of evidence regarding how this should be done. Objectives: This study was carried out to understand the acceptability and usability of a prototype Digital Antibiotic Review Tracking Toolkit and identify modifications required to optimise it ahead of a trial. Methods: The optimisation process involved online semi-structured interviews with a purposive sample of fifteen healthcare professionals recruited from Scotland and England, along with three service users, to gather feedback on the prototype's design, content and delivery. Participants' negative views were specifically sought to identify adaptations needed to ensure that the intervention's components aligned optimally with end-user needs. Data were analysed using Framework Analysis guided by the RE-AIM implementation science framework (Reach, Effectiveness, Adoption, Implementation, and Maintenance) to identify key themes. Results: Participants mostly voiced positive views regarding the prototype, finding it acceptable, feasible and engaging. They also identified concerns relating to its adoption, system functionality, accessibility and maintenance that needed to be addressed. Anticipated low adoption rates were linked to issues surrounding computer literacy. This detailed user feedback informed rapid adjustments to the intervention to enhance its acceptability, perceived future credibility and usability in hospitals. Conclusions: This novel study illustrates how to identify, modify and adapt a digital intervention quickly and efficiently using qualitative iterative methods. Findings highlight the critical importance of contextualising end-user experience with health interventions to facilitate future engagement, uptake, and long-term use. This study also demonstrates how core elements of the MRC framework can be operationalised to help refine prototype digital interventions pre-trial.
Keywords: antibiotic review; antimicrobial resistance; behaviour change; optimisation; person-centred research; qualitative design; user experience.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
How to Implement Digital Clinical Consultations in UK Maternity Care: the ARM@DA Realist Review.Health Soc Care Deliv Res. 2025 May;13(22):1-77. doi: 10.3310/WQFV7425. Health Soc Care Deliv Res. 2025. PMID: 40417997 Review.
-
Technology-enabled CONTACT tracing in care homes in the COVID-19 pandemic: the CONTACT non-randomised mixed-methods feasibility study.Health Technol Assess. 2025 May;29(24):1-24. doi: 10.3310/UHDN6497. Health Technol Assess. 2025. PMID: 40350743 Free PMC article.
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
-
Adapting Safety Plans for Autistic Adults with Involvement from the Autism Community.Autism Adulthood. 2025 May 28;7(3):293-302. doi: 10.1089/aut.2023.0124. eCollection 2025 Jun. Autism Adulthood. 2025. PMID: 40539213
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
References
-
- Public Health England English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report 2023 to 2024. [(accessed on 1 June 2025)]; Available online: https://assets.publishing.service.gov.uk/media/6734e208b613efc3f1823095/....
-
- Skivington K., Matthews L., Simpson S.A., Craig P., Baird J., Blazeby J.M., Boyd K.A., Craig N., French D.P., McIntosh E., et al. A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. Br. Med. J. 2021;374:n2061. doi: 10.1136/bmj.n2061. - DOI - PMC - PubMed
-
- West R., Michie S., Atkins L., Chadwick P., Lorencatto F. Achieving Behaviour Change: A Guide for Local Government and Partners. Public Health England. 2020. [(accessed on 12 May 2024)]; Available online: https://assets.publishing.service.gov.uk/media/5e7b4e85d3bf7f133c923435/....
LinkOut - more resources
Full Text Sources

