Feasibility of Xenogeneic Mitochondrial Transplantation in Neuronal Systems: An Exploratory Study
- PMID: 40724501
- PMCID: PMC12298701
- DOI: 10.3390/life15070998
Feasibility of Xenogeneic Mitochondrial Transplantation in Neuronal Systems: An Exploratory Study
Abstract
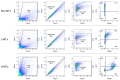
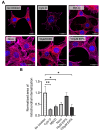
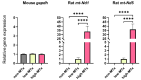
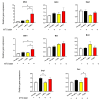
Mitochondrial transplantation (MTx) has emerged as a potential therapeutic approach for diseases associated with mitochondrial dysfunction, yet its scalability and cross-species feasibility remain underexplored. This study aimed to evaluate the dose-dependent uptake and molecular effects of xenogeneic mitochondrial transplantation (xeno-MTx) using rat-derived mitochondria in mouse neuronal systems. HT-22 hippocampal neuronal cells and a murine model of cardiac arrest-induced global cerebral ischemia were used to assess mitochondrial uptake, gene expression, and mitochondrial DNA presence. Donor mitochondria were isolated from rat pectoralis muscle and labeled with MitoTracker dyes. Flow cytometry and confocal microscopy revealed a dose-dependent increase in donor mitochondrial uptake in vitro. Quantitative PCR demonstrated a corresponding increase in rat-specific mitochondrial DNA and upregulation of Mfn2 and Bak1, with no changes in other fusion, fission, or apoptotic genes. Inhibitor studies indicated that mitochondrial internalization may involve actin-dependent macropinocytosis and cholesterol-sensitive endocytic pathways. In vivo, rat mitochondrial DNA was detected in mouse brains post-xeno-MTx, confirming donor mitochondrial delivery to ischemic tissue. These findings support the feasibility of xeno-MTx and its dose-responsive biological effects in neuronal systems while underscoring the need for further research to determine long-term functional outcomes and clinical applicability.
Keywords: cardiac arrest; ischemia–reperfusion; mitochondria; mitochondrial transplantation; neuron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Remote Ischemic Postconditioning Improve Cerebral Ischemia-Reperfusion Injury Induced Cognitive Dysfunction through Suppressing Mitochondrial Apoptosis in Hippocampus via TK/BK/B2R-Mediated PI3K/AKT.Mol Neurobiol. 2025 Aug;62(8):10652-10669. doi: 10.1007/s12035-025-04864-y. Epub 2025 Apr 14. Mol Neurobiol. 2025. PMID: 40229456 Free PMC article.
-
The role of homogenization cycles and Poloxamer 188 on the quality of mitochondria isolated for use in mitochondrial transplantation therapy.Sci Rep. 2025 Jan 27;15(1):3350. doi: 10.1038/s41598-025-86760-y. Sci Rep. 2025. PMID: 39870686 Free PMC article.
-
Mitochondria from huntington´s disease striatal astrocytes are hypermetabolic and compromise neuronal branching.Cell Commun Signal. 2025 Jul 16;23(1):341. doi: 10.1186/s12964-025-02341-6. Cell Commun Signal. 2025. PMID: 40671084 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Sex and gender as predictors for allograft and patient-relevant outcomes after kidney transplantation.Cochrane Database Syst Rev. 2024 Dec 19;12(12):CD014966. doi: 10.1002/14651858.CD014966.pub2. Cochrane Database Syst Rev. 2024. PMID: 39698949
References
-
- Hayashida K., Takegawa R., Shoaib M., Aoki T., Choudhary R.C., Kuschner C.E., Nishikimi M., Miyara S.J., Rolston D.M., Guevara S., et al. Mitochondrial transplantation therapy for ischemia reperfusion injury: A systematic review of animal and human studies. J. Transl. Med. 2021;19:214. doi: 10.1186/s12967-021-02878-3. - DOI - PMC - PubMed
-
- Nakamura E., Aoki T., Endo Y., Kazmi J., Hagiwara J., Kuschner C.E., Yin T., Kim J., Becker L.B., Hayashida K. Organ-Specific Mitochondrial Alterations Following Ischemia-Reperfusion Injury in Post-Cardiac Arrest Syndrome: A Comprehensive Review. Life. 2024;14:477. doi: 10.3390/life14040477. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

