PBPK Modeling of Acetaminophen in Pediatric Populations: Incorporation of SULT Enzyme Ontogeny to Predict Age-Dependent Metabolism and Systemic Exposure
- PMID: 40724601
- PMCID: PMC12300260
- DOI: 10.3390/life15071099
PBPK Modeling of Acetaminophen in Pediatric Populations: Incorporation of SULT Enzyme Ontogeny to Predict Age-Dependent Metabolism and Systemic Exposure
Abstract
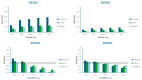
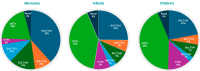
Sulfotransferase (SULT) enzymes contribute significantly to drug metabolism in pediatric patients. The purpose of this study was to develop a PBPK model for acetaminophen (APAP) in pediatric populations that accounts for the ontogeny of SULT isozymes that play a critical role in APAP metabolism. PBPK modeling and simulation were performed using the Simcyp® Simulator. The model incorporated the developmental ontogeny of three key hepatic SULT enzymes: SULT1A1, SULT1A3, and SULT2A1 using "best-fit" ontogeny equations for each isozyme as determined by nonlinear regression analysis of enzyme abundance versus age. PBPK model-simulated pharmacokinetic profiles for APAP captured observed clinical data for systemic exposure (Cmax, AUC) in neonates, infants, and children. SULTS accounted for ~60% APAP metabolism in neonates, with decreased contributions to infants and children. Model sensitivity analysis highlighted the potential for APAP metabolic DDIs, primarily through SULT1A1. The study demonstrates that the impact of SULT enzymes on drug metabolism is significant in neonates, which is an important clinical consideration for APAP. A PBPK model that incorporates SULT ontogeny has the potential to help inform dosing decisions in this special patient population.
Keywords: PBPK modeling; SULT; acetaminophen; neonates; ontogeny; pediatrics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rao C., Shenoy V., Udaykumar P. Potential drug–drug interactions in the pediatric intensive care unit of a tertiary care hospital. J. Pharmacol. Pharmacother. 2019;10:63–68. doi: 10.4103/jpp.JPP_27_19. - DOI
-
- Naji-Talakar S., Sharma S., Martin L.A., Barnhart D., Prasad B. Potential implications of DMET ontogeny on the disposition of commonly prescribed drugs in neonatal and pediatric intensive care units. Expert. Opin. Drug Metab. Toxicol. 2021;17:273–289. doi: 10.1080/17425255.2021.1858051. - DOI - PMC - PubMed
-
- Golchin N., Johnson H., Bakaki P.M., Dawson N., Pestana Knight E.M., Meropol S.B., Liu R., Feinstein J.A., Bolen S.D., Kleinman L.C., et al. Outcome measures in pediatric polypharmacy research: A scoping review. Drugs Ther. Perspect. 2019;35:447–458. doi: 10.1007/s40267-019-00650-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

