Development of INER-PP-F11N as the Peptide-Radionuclide Conjugate Drug Against CCK2 Receptor-Overexpressing Tumors
- PMID: 40724817
- PMCID: PMC12294753
- DOI: 10.3390/ijms26146565
Development of INER-PP-F11N as the Peptide-Radionuclide Conjugate Drug Against CCK2 Receptor-Overexpressing Tumors
Abstract
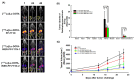
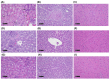
This work aimed to evaluate two albumin affinity structure-containing peptide-radionuclide conjugate drugs, INER-PP-F11N-1 and INER-PP-F11N-2, for the diagnosis/treatment of cholecystokinin receptor subtype 2 (CCK2R)-overexpressing cancers. We developed In-111- and Lu-177-labeled INER-PP-F11N radiopharmaceuticals and compared them with the current PP-F11N to investigate metabolic stability, biodistribution, SPECT/CT imaging, and therapeutic responses in CCK2R-expressing tumor xenograft mice. The metabolic stability of [111In]In/[177Lu]Lu-INER-PP-F11N remained above 90% for up to 144 h after labeling, indicating that the compound is highly stable under in vitro conditions. INER-PP-F11N showed 27% and 11% higher cellular uptake and internalization than PP-F11N, respectively. In vivo SPECT/CT imaging confirmed that INER-PP-F11N could accumulate at the tumor site of mice 24 h after receiving the two radiopharmaceutical agents. Biodistribution analysis revealed a significantly greater tumor uptake and reduced accumulation of INER-PP-F11N in the kidneys compared with PP-F11N. Furthermore, INER-PP-F11N significantly inhibited the growth of the CCK2R-overexpressing tumors in mice. The INER-PP-F11N radiopharmaceutical was superior as a theragnostic agent compared with the current PP-F11N. Our study suggests that INER-PP-F11N may be an innovative radiopharmaceutical agent for CCK2R-overexpressing tumors.
Keywords: CCK2R; INER-PP-F11N; peptide-radionuclide conjugate drug; radiopharmaceutical agent; theragnostic.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
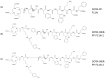


Similar articles
-
Pharmacological inhibition of mTORC1 increases CCKBR-specific tumor uptake of radiolabeled minigastrin analogue [177Lu]Lu-PP-F11N.Theranostics. 2020 Aug 29;10(24):10861-10873. doi: 10.7150/thno.45440. eCollection 2020. Theranostics. 2020. PMID: 33042258 Free PMC article.
-
Targeting of the Cholecystokinin-2 Receptor with the Minigastrin Analog 177Lu-DOTA-PP-F11N: Does the Use of Protease Inhibitors Further Improve In Vivo Distribution?J Nucl Med. 2019 Mar;60(3):393-399. doi: 10.2967/jnumed.118.207845. Epub 2018 Jul 12. J Nucl Med. 2019. PMID: 30002107
-
In Vitro and In Vivo Study of Novel PSMA-Targeted Radioligands: Enhancing Tumor Uptake and Therapeutic Efficacy through Zwitterionization and Albumin-Binding Strategies.Mol Pharm. 2025 Jul 7;22(7):3961-3975. doi: 10.1021/acs.molpharmaceut.5c00214. Epub 2025 Jun 10. Mol Pharm. 2025. PMID: 40494649
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

