Peripheral Leukocyte Syndecan-3 Is Elevated in Alzheimer's Disease: Evidence from a Human Study
- PMID: 40724840
- PMCID: PMC12294132
- DOI: 10.3390/ijms26146587
Peripheral Leukocyte Syndecan-3 Is Elevated in Alzheimer's Disease: Evidence from a Human Study
Abstract
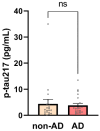
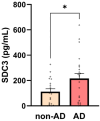
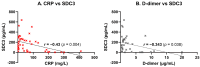
Syndecan-3 (SDC3), a transmembrane heparan sulfate proteoglycan involved in cell signaling and endocytosis, has recently been implicated in the pathogenesis of neurodegenerative disorders. While preclinical studies have demonstrated its role in Alzheimer's disease (AD), its diagnostic relevance in peripheral blood remains unexplored. In this human cohort study, we measured SDC3 expression in peripheral blood mononuclear cells (PBMCs) from 22 clinically diagnosed AD patients and 20 cognitively unimpaired non-AD controls using a custom ELISA. The findings were compared with plasma p-tau217 levels and a panel of systemic laboratory markers. PBMC-expressed SDC3 was significantly elevated in AD patients and moderately correlated with AD status (r = 0.309, p = 0.0465) independent of age. Notably, SDC3 levels were inversely correlated with systemic inflammatory markers, including C-reactive protein (CRP; r = -0.421, p = 0.0055) and D-dimer (r = -0.343, p = 0.038), suggesting an AD-associated immune phenotype distinct from acute-phase or vascular inflammation. Conversely, plasma p-tau217 levels did not significantly differ between groups but correlated with markers of tissue injury and inflammation (LDH, GOT, and ferritin), potentially reflecting systemic influences in non-AD controls. A multivariable logistic regression model incorporating SDC3, p-tau217, and age demonstrated high diagnostic accuracy (AUC = 0.85). These findings identify PBMC-expressed SDC3 as a promising blood-based biomarker candidate for AD, warranting further validation in larger, biomarker-confirmed cohorts.
Keywords: Alzheimer’s disease; biomarker; immune remodeling; neuroinflammation; p-tau217; peripheral blood mononuclear cells; syndecan-3.
Conflict of interest statement
Anett Hudák and Tamás Letoha were employed in Pharmacoidea Ltd. The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Li H., Wang Z. Blood biomarkers for clinical applications in Alzheimer’s disease: A narrative review. NeuroMarkers. 2025;2:100078. doi: 10.1016/j.neumar.2025.100078. - DOI
-
- Hameed S., Fuh J.L., Senanarong V., Ebenezer E.G.M., Looi I., Dominguez J.C., Park K.W., Karanam A.K., Simon O. Role of Fluid Biomarkers and PET Imaging in Early Diagnosis and its Clinical Implication in the Management of Alzheimer’s Disease. J. Alzheimers Dis. Rep. 2020;4:21–37. doi: 10.3233/ADR-190143. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

