Polymorphisms in Base Excision Repair Genes and Association with Multiple Sclerosis in a Pilot Study on a Central European Population
- PMID: 40724862
- PMCID: PMC12295973
- DOI: 10.3390/ijms26146612
Polymorphisms in Base Excision Repair Genes and Association with Multiple Sclerosis in a Pilot Study on a Central European Population
Abstract
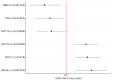
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system characterized by demyelination and neurodegeneration. While its etiology remains unclear, both genetic and environmental factors, including oxidative stress, have been implicated in the development of the disease. The base excision repair (BER) pathway plays a critical role in repairing oxidative DNA damage. This study investigated the association between polymorphisms in BER-related genes and MS susceptibility in a Central European population. Ten SNPs across seven BER genes were genotyped in 102 patients with MS and 118 healthy controls. Six SNPs were significantly associated with MS. Increased risk was observed for rs25478 in XRCC1 (OR = 2.37, 95% CI: 1.44-3.91, p < 0.0001), rs3087404 in SMUG1 (OR = 2.80, 95% CI: 1.49-5.26, p = 0.0012), and rs3219493 in MUTYH (OR = 2.23, 95% CI: 1.35-3.67, p = 0.0018). Conversely, reduced risk was associated with rs2307293 in MBD4 (OR = 0.42, 95% CI: 0.23-0.78, p = 0.006), rs3219489 in MUTYH (OR = 0.55, 95% CI: 0.31-0.97, p = 0.038), and rs4135054 in TDG (OR = 0.52, 95% CI: 0.29-0.94, p = 0.031). Haplotype analysis was performed for SNPs in strong linkage disequilibrium. Only rs3219489 and rs3219472 within the MUTYH gene showed strong LD (r2 = 0.90), justifying haplotype-based analysis. Among four inferred haplotypes, the rare G-C haplotype was significantly associated with reduced MS risk (Score = -2.10, p = 0.035), suggesting a protective effect of this allele combination. Other SNPs not in LD were analyzed using a multivariable logistic regression model. Significant associations with decreased MS risk were found for rs1052133 in OGG1 (OR = 0.57, p = 0.043), rs2307293 in MBD4 (OR = 0.16, p = 0.010), and rs4135054 in TDG (OR = 0.38, p < 0.001), while rs3087404 in SMUG1 increased MS risk (OR = 1.98, p = 0.013). These results suggest that genetic variation in BER genes, including both single SNP effects and haplotypes, contributes to MS susceptibility. Further studies are warranted to explore the functional consequences of these variants and validate findings in larger, independent cohorts.
Keywords: base excision repair; gene polymorphisms; multiple sclerosis.
Conflict of interest statement
The authors declare that they have no conflicts of interest. The funders had no role in the study’s design, data collection, analysis, interpretation, manuscript writing, or publishing of the results.
Figures
Similar articles
-
Oxidative DNA Damage and Repair Dynamics in Multiple Sclerosis: Insights from Comet Assay Kinetics, Base Excision Repair Gene Expression, and Genotype Analysis.Biomolecules. 2025 May 24;15(6):756. doi: 10.3390/biom15060756. Biomolecules. 2025. PMID: 40563398 Free PMC article.
-
A Case-Control Study on Combined Effects of Base Excision Repair and Nucleotide Excision Repair Gene Polymorphisms in Gastrointestinal Cancer Susceptibility.Asian Pac J Cancer Prev. 2025 Aug 1;26(8):2909-2917. doi: 10.31557/APJCP.2025.26.8.2909. Asian Pac J Cancer Prev. 2025. PMID: 40849707
-
Association between polymorphisms in genes related to DNA base-excision repair with risk and prognosis of oropharyngeal squamous cell carcinoma.J Cancer Res Clin Oncol. 2016 Sep;142(9):1917-26. doi: 10.1007/s00432-016-2202-8. Epub 2016 Jul 2. J Cancer Res Clin Oncol. 2016. PMID: 27372710 Free PMC article.
-
Impact of DNA polymorphisms in key DNA base excision repair proteins on cancer risk.Hum Exp Toxicol. 2012 Oct;31(10):981-1005. doi: 10.1177/0960327112444476. Epub 2012 Sep 27. Hum Exp Toxicol. 2012. PMID: 23023028 Free PMC article.
-
Association between X-ray repair cross-complementing group 1(XRCC1) Arg399Gln polymorphism and endometriosis: A systematic review and meta-analysis.Eur J Obstet Gynecol Reprod Biol. 2017 Nov;218:12-20. doi: 10.1016/j.ejogrb.2017.09.011. Epub 2017 Sep 14. Eur J Obstet Gynecol Reprod Biol. 2017. PMID: 28926725
References
-
- Portaccio E., Magyari M., Havrdova E.K., Ruet A., Brochet B., Scalfari A., Di Filippo M., Tur C., Montalban X., Amato M.P. Multiple sclerosis: Emerging epidemiological trends and redefining the clinical course. Lancet Reg. Health Eur. 2024;44:100977. doi: 10.1016/j.lanepe.2024.100977. - DOI - PMC - PubMed
-
- Multiple Sclerosis International Federation—Atlas of MS—3rd Edition. [(accessed on 25 February 2025)]. Available online: https://www.atlasofms.org/map/poland/epidemiology/number-of-people-with-ms.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

