The Role of Lactylation in Virus-Host Interactions
- PMID: 40724863
- PMCID: PMC12295935
- DOI: 10.3390/ijms26146613
The Role of Lactylation in Virus-Host Interactions
Abstract
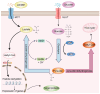
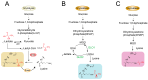
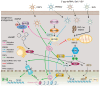
Lactylation, a novel form of post-translational modifications (PTMs) of protein, particularly within histone proteins, has recently gained attention for its role in regulating gene expression and cellular processes. In recent years, lactylation has been widely studied in cancer, immune diseases, neurological diseases, cardiovascular diseases, metabolic diseases, etc. Increasing evidence now suggests that lactylation also plays a significant role in the host's innate immune response to viruses. Lactylation influences fundamental cellular functions, including transcriptional regulation, signal transduction, cell proliferation and differentiation. It affects protein behavior by modulating their function, stability, subcellular localization and interactions. Studies have shown that many viral infections promote lactate production through enhanced glycolysis, a process that facilitates viral replication. Given that innate immunity serves as the host's first line of defense against pathogenic invasion, understanding how lactylation regulates antiviral responses offers promising avenues for the development of diagnostic tools and therapeutic strategies against viral diseases. In this review, we provide a comprehensive overview of recent research on the role of lactylation in viral-host interactions.
Keywords: innate immunity; lactylation; post-translational modifications (PTMs); viral infection; virus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Krishnan S., Nordqvist H., Ambikan A.T., Gupta S., Sperk M., Svensson-Akusjärvi S., Mikaeloff F., Benfeitas R., Saccon E., Ponnan S.M., et al. Metabolic Perturbation Associated with COVID-19 Disease Severity and SARS-CoV-2 Replication. Mol. Cell Proteom. 2021;20:100159. doi: 10.1016/j.mcpro.2021.100159. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

