Gray-Horse Melanoma-A Wolf in Sheep's Clothing
- PMID: 40724880
- PMCID: PMC12295847
- DOI: 10.3390/ijms26146620
Gray-Horse Melanoma-A Wolf in Sheep's Clothing
Abstract
Malignant melanoma (MM) affects not only humans but also animals, with gray horses being particularly predisposed to acquiring the disease. Multiomics have greatly advanced the understanding of human MM. In contrasty little is known regarding the pathogenesis of gray-horse melanoma and the unique phenomenon of melanoma "dormancy" in some animals. To help close this gap in knowledge, melanoma tissue and intact skin collected from gray horses were subjected to transcriptome analysis using RNAseq. In the next step, cultured primary tumor cells and normal skin fibroblasts were established from gray horses, and their protein expression profiles were determined. The obtained data unambiguously identified gray-horse melanoma (ghM) as a malignant tumor, as reflected by the overrepresentation of pathways typically activated in human melanoma and other human cancers. These included the RAS/RAF/MAPK, the IRS/IGF1R, and the PI3K/AKT signaling networks. In addition, the obtained data suggest that the key molecules RAC1, RAS, and BRAF, which are frequently mutated in human melanoma, may also contain activating mutations in ghM, whilst PTEN may harbor loss-of-function mutations. This issue will be subject to downstream analyses determining the mutational status in ghM to further advance the understanding of this frequent disease in gray horses.
Keywords: RNAseq; gray-horse melanoma; melanoma; primary melanoma cells; proteomics; tumor tissue.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

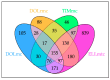
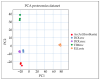
References
-
- Müller S., Wanke R., Distl O. Inheritance of melanocytic lesions and their association with the white colour phenotype in miniature swine. J. Anim. Breed. Genet. 2001;118:275–283. doi: 10.1046/j.1439-0388.2001.00280.x. - DOI
-
- Scott D.W., Miller W.H.J. Equine Dermatology. 2nd ed. Elsevier Saunders; Maryland Heights, MO, USA: 2011. Melanoma; pp. 506–508.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

