Optimal Horseshoe Crab Blood Collection Solution That Inhibits Cellular Exocytosis and Improves Production Yield of Limulus Amoebocyte Lysate for Use in Endotoxin Tests
- PMID: 40724892
- PMCID: PMC12295968
- DOI: 10.3390/ijms26146642
Optimal Horseshoe Crab Blood Collection Solution That Inhibits Cellular Exocytosis and Improves Production Yield of Limulus Amoebocyte Lysate for Use in Endotoxin Tests
Abstract
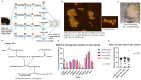
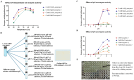
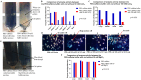
Limulus amoebocyte lysate (LAL) assays have emerged as among the most effective approaches for detecting endotoxins and fungi in vitro since they were first tested 50 years ago. Although detailed protocols are publicly available, conventional LAL collection methods (3% sodium chloride) waste as much as 80% of the total LAL during blood accumulation, confirming the incompatibility of these methods with the lasting survival of the American horseshoe crab. For this reason, new implementations of blood collection-suspension buffer combinations are critical. Here, we evaluated the ability of different blood collection solutions to inhibit exocytosis and subsequently treated the cells with CaCl2 to stimulate exocytosis and improve the yield of LAL. Two test methods, chromogenic and turbidimetric tests for LAL activity, were evaluated. Crabs were bled during the bleeding season. The crab blood samples were collected with the following blood collection solutions: citric acid buffer, malic acid buffer, PBS buffer, and PBS-caffeine buffer. The cell pellets were washed with 3% NaCl and subsequently resuspended in LRW or CaCl2 to facilitate degranulation. Both the chromogenic test and the turbidimetric assay were used to evaluate the LAL enzyme activity. Citric acid buffer, malic acid buffer, PBS buffer, and PBS-caffeine buffer blocked exocytosis, resulting in the high yields of LAL. There was no observable effect on the activity output of crab size via a chromogenic test with PBS-caffeine buffer during the bleeding season. This protocol substantially benefited prior processes, as the PBS-caffeine collection mixture decreased amoebocyte aggregation/clot formation during processing. Furthermore, we evaluated the specific biochemical parameters of PBS-caffeine-derived LAL. We developed an accessible, promising phosphate-caffeine-based blood collection buffer that prevents amoebocyte degranulation during blood collection, maximizing the LAL yield. Moreover, our analysis revealed that phosphate-caffeine-derived LAL is uniquely adaptable to compatibility with chromogenic and turbidimetric assay techniques. By employing this method for LAL blood extraction, our same-cost approach fostered significantly greater LAL yields, simultaneously ensuring a healthy limulus polyphemus population.
Keywords: PBS–caffeine; chromogenic assay; endotoxin; exocytosis; limulus amoebocyte lysate (LAL) assays.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Tamura H., Reich J., Nagaoka I. Outstanding Contributions of LAL Technology to Pharmaceutical and Medical Science: Review of Methods, Progress, Challenges, and Future Perspectives in Early Detection and Management of Bacterial Infections and Invasive Fungal Diseases. Biomedicines. 2021;9:536. doi: 10.3390/biomedicines9050536. - DOI - PMC - PubMed
-
- Bloos F., Held J., Kluge S., Simon P., Kogelmann K., de Heer G., Kuhn S.-O., Jarczak D., Motsch J., Hempel G., et al. (1→ 3)-β-D-Glucan-guided antifungal therapy in adults with sepsis: The CandiSep randomized clinical trial. Intensive Care Med. 2022;48:865–875. doi: 10.1007/s00134-022-06733-x. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

