Simulated Microgravity Attenuates Stretch Sensitivity of Mechanically Gated Channels in Rat Ventricular Myocytes
- PMID: 40724903
- PMCID: PMC12294224
- DOI: 10.3390/ijms26146653
Simulated Microgravity Attenuates Stretch Sensitivity of Mechanically Gated Channels in Rat Ventricular Myocytes
Abstract
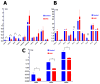
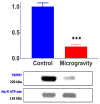
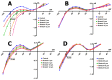
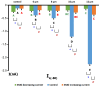
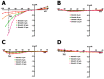
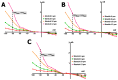
Cardiomyocytes, similarly to cells in various tissues, are responsive to mechanical stress of all types, which is reflected in the significant alterations to their electrophysiological characteristics. This phenomenon, known as mechanoelectric feedback, is based on the work of mechanically gated channels (MGCs) and mechano-sensitive channels (MSCs). Since microgravity (MG) in space, as well as simulated microgravity (SMG), changes the morphological and physiological properties of the heart, it was assumed that this result would be associated with a change in the expression of genes encoding MGCs and MSCs, leading to a change in the synthesis of channel proteins and, ultimately, a change in channel currents during cell stretching. In isolated ventricular cardiomyocytes of rats exposed to SMG for 14 days, the amount of MGCs and MSCs gene transcripts was studied using the RNA sequencing method by normalizing the amount of "raw" reads using the Transcripts Per Kilobase Million (TPM) method. Changes in the level of channel protein, using the example of the MGCs TRPM7, were assessed by the Western blot method, and changes in membrane ion currents in the control and during cardiomyocyte stretching were assessed by the patch-clamp method in the whole-cell configuration. The data obtained demonstrate that SMG results in a multidirectional change in the expression of genes encoding various MGCs and MSCs. At the same time, a decrease in the TPM of the MGCs TRPM7 gene leads to a decrease in the amount of TRPM7 protein. The resulting redistribution in the synthesis of most channel proteins leads to a marked decrease in the sensitivity of the current through MGCs to cell stretching and, ultimately, to a change in the functioning of the heart.
Keywords: TRPM7; mechanosensitive channels; rat; simulated microgravity; stretch; ventricular myocytes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Simulated Microgravity Changes the Number of Mechanically Gated and Mechanosensitive Ion Channels Genes Transcripts in Rat Ventricular Cardiomyocytes.Dokl Biochem Biophys. 2023 Oct;512(1):251-255. doi: 10.1134/S1607672923700369. Epub 2023 Dec 13. Dokl Biochem Biophys. 2023. PMID: 38093125
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Disturbed Repolarization-Relaxation Coupling During Acute Myocardial Ischemia Permits Systolic Mechano-Arrhythmogenesis.Circ Res. 2025 Jul 18;137(3):363-382. doi: 10.1161/CIRCRESAHA.124.326057. Epub 2025 Jun 2. Circ Res. 2025. PMID: 40452589 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

