Organ-Specific Small Protein Networks in 100 kDa Ultrafiltrates: Functional Analysis and Implications for Neuroregenerative Medicine
- PMID: 40724909
- PMCID: PMC12294768
- DOI: 10.3390/ijms26146659
Organ-Specific Small Protein Networks in 100 kDa Ultrafiltrates: Functional Analysis and Implications for Neuroregenerative Medicine
Abstract
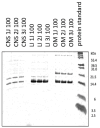
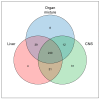
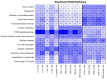
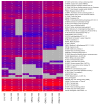
In this research, the proteomic landscape of 100 kDa protein extract sourced from rabbit brain was compared to extracts from liver and from organ mixture (OM). Our aim was to compare the efficacy of Nanomised Organo Peptides (NOP) ultrafiltrates from two different tissues and a tissue mixture for inducing neurite outgrowth, and subsequently to identify the molecular networks and proteins that could explain such effects. Proteins were isolated by gentle homogenization followed by crossflow ultrafiltration. Proteomic evaluation involved gel electrophoresis, complemented by mass spectrometry and bioinformatics. GO (Gene Ontology) and protein analysis of the mass spectrometry results identified a diverse array of proteins involved in critical specific biological functions, including neuronal development, regulation of growth, immune response, and lipid and metal binding. Data from this study are accessible from the ProteomeXchange repository (identifier PXD051701). Our findings highlight the presence of small proteins that play key roles in metabolic processes and biosynthetic modulation. In vitro outgrowth experiments with neural stem cells (NSCs) showed that 100 kDa protein extracts from the brain resulted in a greater increase in neurite length compared to the liver and organ mixture extracts. The protein networks identified in the NOP ultrafiltrates may significantly improve biological therapeutic strategies related to neural differentiation and outgrowth. This comprehensive proteomic analysis of 100 kDa ultrafiltrates revealed a diverse array of proteins involved in key biological processes, such as neuronal development, metabolic regulation, and immune response. Brain-specific extracts demonstrated the capacity to promote neurite outgrowth in NSCs, suggesting potential application for neuroregenerative therapies. Our findings highlight the potential of small proteins and organ-specific proteins in the development of novel targeted treatments for various diseases, particularly those related to neurodegeneration and aging.
Keywords: organ specificity; proteomics; small proteins; ultrafiltration.
Conflict of interest statement
Authors M.K.S.C. and M.B.F.W. affiliated with Stellar Biomolecular Research GmbH and EW European Wellness International GmbH are not involved in formal analysis and investigation. Author J.P.S. is an employee of the company Reviva s.r.o. Author C.B. was an employee of the company MicroDiscovery GmbH at the time of preparing and writing of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures






References
-
- Zhou J., Liu K., Bauer C., Bendner G., Dietrich H., Slivka J.P., Wink M., Wong M.B.F., Chan M.K.S., Skutella T. Modulation of Cellular Senescence in HEK293 and HepG2 Cells by Ultrafiltrates UPla and ULu Is Partly Mediated by Modulation of Mitochondrial Homeostasis under Oxidative Stress. Int. J. Mol. Sci. 2023;24:6748. doi: 10.3390/ijms24076748. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

