Biochemical Insights into the Effects of a Small Molecule Drug Candidate on Imatinib-Induced Cardiac Inflammation
- PMID: 40724911
- PMCID: PMC12294382
- DOI: 10.3390/ijms26146661
Biochemical Insights into the Effects of a Small Molecule Drug Candidate on Imatinib-Induced Cardiac Inflammation
Abstract
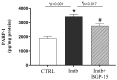
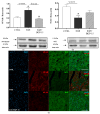
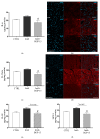
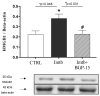
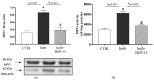
BGP-15, a poly(ADP-ribose) polymerase-1 (PARP-1) inhibitor exerts cardioprotective effects; however, the underlying mechanisms remain unclear. Therefore, our study aimed to investigate the effects of BGP-15 on the imatinib (Imtb)-induced cardiac inflammation at the biochemical level. Male rats were divided to control, Imtb-treated (60 mg/kg/day for 14 days), and Imtb + BGP-15-treated animals. In this group Imtb was co-administered with BGP-15 at the dose of 10 mg/kg/day. At the end of the experiment, nuclear factor-kappa B/p65 (NF-κB/p65), nuclear transcription factor erythroid-2 related factor (Nrf2), heme oxygenase-1 (HO-1), high mobility group box 1 (HMGB1), and myeloperoxidase (MPO) were measured by Western blot. Chemokine and interleukins (ILs) were determined by Legendplex. Additionally, cardiac specific changes were visualized by immunohistochemistry. We demonstrated that Imtb increased NF-κB/p65, IL-6, IL-1β, IL-18, MCP-1, HMGB1, as well as the expression and activity of MPO. Conversely, the expressions of antioxidant Nrf2 and HO-1 were decreased. Administration of BGP-15 effectively mitigated these inflammatory alterations by significantly reducing pro-inflammatory cytokines and MPO activity, while simultaneously restoring and enhancing the levels of Nrf2 and HO-1, thereby promoting antioxidant defenses. The immunohistochemical staining further supported these biochemical changes. Our study provides new and comprehensive biochemical insight for managing Imtb-induced inflammatory responses via BGP-15-induced PARP1 inhibition.
Keywords: BGP-15; PARP-1; imatinib; inflammation.
Conflict of interest statement
Author Gyöngyi Kis was employed by the company Creative Laboratory Ltd. She and the other authors declare no conflict of interest.
Figures

Similar articles
-
Effectiveness of the fruit of Rosa odorata sweet var. gigantea (Coll. et Hemsl.) Rehd. et Wils in the protection and the healing of ethanol-induced rat gastric mucosa ulcer based on Nrf2/NF-κB pathway regulation.J Ethnopharmacol. 2022 Jan 10;282:114626. doi: 10.1016/j.jep.2021.114626. Epub 2021 Sep 10. J Ethnopharmacol. 2022. PMID: 34517064
-
Buzhong Yiqi decoction improves inflammation and oxidative damage in autoimmune thyroiditis by inhibiting apoptosis via the SIRT1-Mediated Nrf2/NF-κB axis.J Ethnopharmacol. 2025 Jul 24;351:119967. doi: 10.1016/j.jep.2025.119967. Epub 2025 May 11. J Ethnopharmacol. 2025. PMID: 40360040
-
The impact of Nrf2/HO-1, PD-L1/Bax/Bcl-2 and TNF-α/Il-6 signaling pathways in the ameliorative role of cordycepin against acrylamide-induced cardiac toxicity in rats.Sci Rep. 2025 Jul 2;15(1):23599. doi: 10.1038/s41598-025-08525-x. Sci Rep. 2025. PMID: 40604049 Free PMC article.
-
NTP Developmental and Reproductive Toxicity Technical Report on the Prenatal Development Studies of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine (CASRN 95737-68-1) in Sprague Dawley (Hsd:Sprague Dawley® SD®) Rats and New Zealand White (Hra:NZW SPF) Rabbits: DART Report 07 [Internet].Research Triangle Park (NC): National Toxicology Program; 2022 Jan. Research Triangle Park (NC): National Toxicology Program; 2022 Jan. PMID: 35593777 Free Books & Documents. Review.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
References
-
- World Heath Organization. [(accessed on 7 July 2025)]. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--a....
-
- Chianca M., Fabiani I., Del Franco A., Grigoratos C., Aimo A., Panichella G., Giannoni A., Castiglione V., Gentile F., Passino C., et al. Management and treatment of cardiotoxicity due to anticancer drugs: 10 questions and answers. Eur. J. Prev. Cardiol. 2022;29:2163–2172. doi: 10.1093/eurjpc/zwac170. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

