Laser-Induced Dimeric Photoproducts of Chlorpromazine: LC-MS Identification and Molecular Docking Evidence of Enhanced Anticancer Potential
- PMID: 40724916
- PMCID: PMC12294483
- DOI: 10.3390/ijms26146668
Laser-Induced Dimeric Photoproducts of Chlorpromazine: LC-MS Identification and Molecular Docking Evidence of Enhanced Anticancer Potential
Abstract
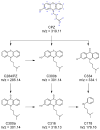
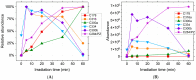
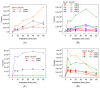
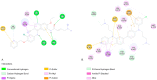
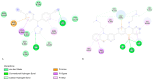
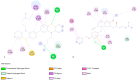
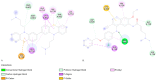
Breast cancer treatments, such as chemotherapy, radiation, and surgery, often face significant limitations, highlighting the need for more effective and targeted therapies. Here, we investigate the potential of 266 nm laser irradiation of chlorpromazine as a novel approach to develop new antitumoral compounds. We identify six chlorpromazine photocompounds with masses in the range of 178-334 u, along with several dimeric compounds with masses between 566 and 600 u, using an HPLC-MS. In silico approaches assess their pharmacokinetic and pharmacodynamic properties while comparing their toxicity with the parent compound. Molecular docking simulations indicate that some photoproducts have a low estimated free energy of binding to cancer-related targets, suggesting enhanced therapeutic potential compared to chlorpromazine. Additionally, ADME-Tox predictions indicate that these photoproducts may have pharmacokinetic and toxicity profiles similar to chlorpromazine. Overall, this study highlights that laser-generated chlorpromazine photoproducts exhibit enhanced biological activity to breast cancer-related targets compared to chlorpromazine while maintaining a similar ADME-Tox profile.
Keywords: ADME-Tox predictions; HPLC-MS; MS2; breast cancer; chlorpromazine; laser irradiation; molecular docking.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Interventions for fertility preservation in women with cancer undergoing chemotherapy.Cochrane Database Syst Rev. 2025 Jun 19;6(6):CD012891. doi: 10.1002/14651858.CD012891.pub2. Cochrane Database Syst Rev. 2025. PMID: 40536056 Review.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Vasileiou M., Papageorgiou S., Nguyen N.P. Current Advancements and Future Perspectives of Immunotherapy in Breast Cancer Treatment. Immuno. 2023;3:195–216. doi: 10.3390/immuno3020013. - DOI
-
- Comşa Ş., Cîmpean A.M., Raica M. The Story of MCF-7 Breast Cancer Cell Line: 40 Years of Experience in Research. Anticancer Res. 2015;35:3147–3154. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

