LightSpot Fluorescent Conjugates as Highly Efficient Tools for Lysosomal P-gp Quantification in Olaparib-Treated Triple-Negative Breast Cancer Cells
- PMID: 40724925
- PMCID: PMC12294673
- DOI: 10.3390/ijms26146675
LightSpot Fluorescent Conjugates as Highly Efficient Tools for Lysosomal P-gp Quantification in Olaparib-Treated Triple-Negative Breast Cancer Cells
Abstract
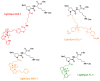
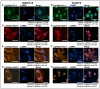
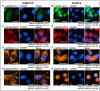
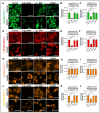
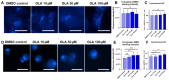
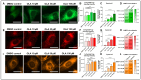
P-glycoprotein (P-gp) is a key element of cancer treatment resistance, actively extruding cytotoxic drugs from cells and diminishing their efficacy. While its role at the plasma membrane is well established, its intracellular localization, particularly on lysosomes, is increasingly recognized as a critical contributor to drug resistance. This study investigates four innovative LightSpot fluorescent compounds to detect and quantify both membrane and lysosomal P-gp in Triple-Negative Breast Cancer (TNBC) SUM1315 and DU4475 cell lines. Results highlighted lysosomal P-gp staining by the LightSpot-FL-1, LightSpot-BrX-1, and LightSpot-BdO-1 fluorescent compounds (Mander's coefficients > 0.8 overlapping with LAMP2 immunostaining). After both cell lines were exposed to Olaparib, a significant increase in P-gp expression level and lysosomal distribution of P-gp was detected. Indeed, after 100 µM Olaparib exposure, LightSpot-FL-1 allowed us to quantify an increase in P-gp-positive lysosome number of 1293 and 334% for SUM1315 and DU4475 cells, respectively, compared to the control. Findings suggest that P-gp may relocate to lysosomes upon drug exposure, highlighting a dual resistance mechanism involving both membrane and lysosomal P-gp. This study demonstrated the potential of LightSpot fluorescent compounds to evaluate P-gp-mediated cell resistance to treatment and emphasized the need to assess global cell P-gp expression to improve cancer diagnosis.
Keywords: LightSpot fluorescent conjugates; lysosomal P-gp; multidrug resistance; permeability-glycoprotein; triple-negative breast cancer cell lines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
LightSpot®-FL-1 Fluorescent Probe: An Innovative Tool for Cancer Drug Resistance Analysis by Direct Detection and Quantification of the P-glycoprotein (P-gp) on Monolayer Culture and Spheroid Triple Negative Breast Cancer Models.Cancers (Basel). 2021 Aug 11;13(16):4050. doi: 10.3390/cancers13164050. Cancers (Basel). 2021. PMID: 34439204 Free PMC article.
-
Co-Inhibition of PARP and STAT3 as a Promising Approach for Triple-Negative Breast Cancer.Biomolecules. 2025 Jul 17;15(7):1035. doi: 10.3390/biom15071035. Biomolecules. 2025. PMID: 40723906 Free PMC article.
-
An update on cancer stem cell survival pathways involved in chemoresistance in triple-negative breast cancer.Future Oncol. 2025 Mar;21(6):715-735. doi: 10.1080/14796694.2025.2461443. Epub 2025 Feb 12. Future Oncol. 2025. PMID: 39936282 Review.
-
P-gp quantification for cell resistance studies in leukemia cells using the innovative LightSpot-FL-1 fluorescent conjugate.Sci Rep. 2025 May 25;15(1):18173. doi: 10.1038/s41598-025-03370-4. Sci Rep. 2025. PMID: 40414975 Free PMC article.
-
Olaparib Monotherapy or in Combination with Abiraterone for the Treatment of Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) and a BRCA Mutation.Target Oncol. 2025 May;20(3):445-466. doi: 10.1007/s11523-025-01146-4. Epub 2025 May 21. Target Oncol. 2025. PMID: 40397306 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

