Use of Radiomics in Characterizing Tumor Hypoxia
- PMID: 40724929
- PMCID: PMC12294197
- DOI: 10.3390/ijms26146679
Use of Radiomics in Characterizing Tumor Hypoxia
Abstract
Tumor hypoxia involves limited oxygen supply within the tumor microenvironment and is closely associated with aggressiveness, metastasis, and resistance to common cancer treatment modalities such as chemotherapy and radiotherapy. Traditional methodologies for hypoxia assessment, such as the use of invasive probes and clinical biomarkers, are generally not very suitable for routine clinical applications. Radiomics provides a non-invasive approach to hypoxia assessment by extracting quantitative features from medical images. Thus, radiomics is important in diagnosis and the formulation of a treatment strategy for tumor hypoxia. This article discusses the various imaging techniques used for the assessment of tumor hypoxia including magnetic resonance imaging (MRI), positron emission tomography (PET), and computed tomography (CT). It introduces the use of radiomics with machine learning and deep learning for extracting quantitative features, along with its possible clinical use in hypoxic tumors. This article further summarizes the key challenges hindering the clinical translation of radiomics, including the lack of imaging standardization and the limited availability of hypoxia-labeled datasets. It also highlights the potential of integrating radiomics with multi-omics to enhance hypoxia visualization and guide personalized cancer treatment.
Keywords: deep learning; machine learning; medical imaging; non-invasive assessment; radiomics; tumor hypoxia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
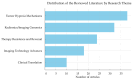


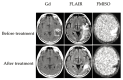
References
-
- Simoes-Sousa S., Littler S., Thompson S.L., Minshall P., Whalley H., Bakker B., Belkot K., Moralli D., Bronder D., Tighe A., et al. The p38alpha Stress Kinase Suppresses Aneuploidy Tolerance by Inhibiting Hif-1alpha. Cell Rep. 2018;25:749–760 e746. doi: 10.1016/j.celrep.2018.09.060. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

