Citrate Transporter Expression and Localization: The Slc13a5Flag Mouse Model
- PMID: 40724960
- PMCID: PMC12294767
- DOI: 10.3390/ijms26146707
Citrate Transporter Expression and Localization: The Slc13a5Flag Mouse Model
Abstract
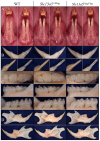
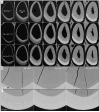
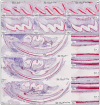
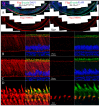
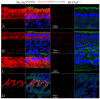
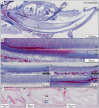
The sodium-citrate cotransporter (NaCT) plays a crucial role in citrate transport during amelogenesis. Mutations in the SLC13A5 gene, which encodes the NaCT, cause early infantile epileptic encephalopathy 25 and amelogenesis imperfecta. We analyzed developing pig molars and determined that the citrate concentrations in secretory- and maturation-stage enamel are both 5.3 µmol/g, with about 95% of the citrate being bound to mineral. To better understand how citrate might enter developing enamel, we developed Slc13a5Flag reporter mice that express NaCT with a C-terminal Flag-tag (DYKDDDDK) that can be specifically and accurately recognized by commercially available anti-Flag antibodies. The 24-base Flag coding sequence was located immediately upstream of the natural translation termination codon (TAG) and was validated by Sanger sequencing. The general development, physical activities, and reproductive outcomes of this mouse strain were comparable to those of the C57BL/6 mice. No differences were detected between the Slc13a5Flag and wild-type mice. Tooth development was extensively characterized using dissection microscopy, bSEM, light microscopy, in situ hybridization, and immunohistochemistry. Tooth formation was not altered in any detectable way by the introduction of the Flag. The Slc13a5Flag citrate transporter was observed on all outer membranes of secretory ameloblasts (distal, lateral, and proximal), with the strongest signal on the Tomes process, and was detectable in all but the distal membrane of maturation-stage ameloblasts. The papillary layer also showed positive immunostaining for Flag. The outer membrane of odontoblasts stained stronger than ameloblasts, except for the odontoblastic processes, which did not immunostain. As NaCT is thought to only facilitate citrate entry into the cell, we performed in situ hybridization that showed Ank is not expressed by secretory- or maturation-stage ameloblasts, ruling out that ANK can transport citrate into enamel. In conclusion, we developed Slc13a5Flag reporter mice that provide specific and sensitive localization of a fully functional NaCT-Flag protein. The localization of the Slc13a5Flag citrate transporter throughout the ameloblast membrane suggests that either citrate enters enamel by a paracellular route or NaCT can transport citrate bidirectionally (into or out of ameloblasts) depending upon local conditions.
Keywords: amelogenesis; enamel mineralization; protein localization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

