Oxidative Stress in HIV-Associated Neurodegeneration: Mechanisms of Pathogenesis and Therapeutic Targets
- PMID: 40724974
- PMCID: PMC12295029
- DOI: 10.3390/ijms26146724
Oxidative Stress in HIV-Associated Neurodegeneration: Mechanisms of Pathogenesis and Therapeutic Targets
Abstract
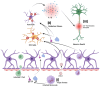
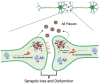
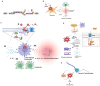
Treatment for HIV infection has become more manageable due to advances in combination antiretroviral therapy (cART). However, HIV still significantly affects the central nervous system (CNS) in infected individuals, even with effective plasma viral suppression, due to persistent viral reservoirs and chronic neuroinflammation. This ongoing inflammation contributes to the development of HIV-associated neurocognitive disorders (HANDs), including dementia and Alzheimer's disease-like pathology. These complications are particularly prevalent among the aging population with HIV. This review aims to provide a comprehensive overview of HAND, with a focus on the contribution of oxidative stress induced by HIV-mediated reactive oxygen species (ROS) production through viral proteins such as gp120, Tat, Nef, Vpr, and reverse transcriptase. In addition, we discuss current and emerging therapeutic interventions targeting HAND, including antioxidant strategies and poly (ADP-ribose) polymerase (PARP) inhibitors. These are potential adjunctive approaches to mitigate neuroinflammation and oxidative damage in the CNS.
Keywords: Alzheimer’s Disease (AD); HIV-associated neurocognitive disorders (HAND); central nervous system (CNS); human immunodeficiency virus type 1 (HIV-1); reactive oxygen species (ROS).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

