Synergistic Production of Lycopene and β-Alanine Through Engineered Redox Balancing in Escherichia coli
- PMID: 40724976
- PMCID: PMC12294996
- DOI: 10.3390/ijms26146727
Synergistic Production of Lycopene and β-Alanine Through Engineered Redox Balancing in Escherichia coli
Abstract
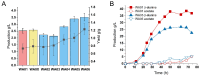
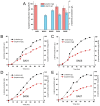
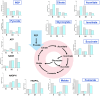
The production of β-alanine from fatty acid feedstocks presents a promising synthetic strategy due to its high carbon yield. However, the excessive reducing power generated during fatty acid utilization disrupts cellular redox balance, adversely affecting metabolism and limiting the efficiency and final yield of β-alanine production. To address this challenge, we engineered a co-production system in which excess reducing equivalents generated during fatty acid β-oxidation and β-alanine biosynthesis were consumed by growth-coupled lycopene biosynthesis. The resulting dual-pathway strain, SA01, achieved 44.78 g/L β-alanine and 3.07 g/L lycopene in bioreactor fermentation, representing a 21.45% increase in β-alanine production compared to the β-alanine-producing strain WA01, and a 74.43% increase in lycopene production compared to the lycopene-producing strain LA01. Further optimization in strain SA06, involving cofactor engineering to shift redox flow from NADH to NADPH, enhanced the titers to 52.78 g/L β-alanine and 3.61 g/L lycopene. Metabolite analysis confirmed a decrease in intracellular NADH and FADH2 levels in SA06, indicating restoration of redox balance during the late fermentation phase. Additional improvements in the fermentation process, including gradual carbon source switching, optimization of the induction strategy, and fine-tuning of conditions during both growth and bioconversion phases, resulted in further increases in product titers, reaching 72 g/L β-alanine and 6.15 g/L lycopene. This study offers valuable insights into the development of microbial co-production systems, highlighting the critical role of dynamic cofactor and redox balance management, as well as process optimization, in improving production efficiency.
Keywords: Escherichia coli; lycopene; metabolic engineering; redox balance; β-alanine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Orfali D.M., Meramo S., Sukumara S. Ranking economic and environmental performance of feedstocks used in bio-based production systems. Curr. Res. Biotechnol. 2025;9:100275. doi: 10.1016/j.crbiot.2025.100275. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

