Radionuclides Landscape in Prostate Cancer Theranostics
- PMID: 40724999
- PMCID: PMC12296053
- DOI: 10.3390/ijms26146751
Radionuclides Landscape in Prostate Cancer Theranostics
Abstract
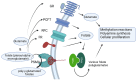
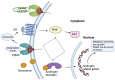
Prostate cancer, a malignancy of significant prevalence, affects approximately half a million men in Europe, with one in twelve males receiving a diagnosis before reaching the age of 75. Radiotheranostics represents a paradigm shift in prostate cancer treatment, leveraging radionuclides for diagnostic and therapeutic applications, with PSMA emerging as the primary molecular target. Regulatory bodies have approved various PSMA-targeted radiodiagnostic agents, such as [18F]DCFPyL (PYLARIFY®, Lantheus Holdings), [18F]rhPSMA-7.3 (POSLUMA®, Blue Earth Diagnostics), and [68Ga]Ga-PSMA-11 (LOCAMETZ®, Novartis/ILLUCCIX®, Telix Pharmaceuticals), as well as therapeutic agents like [177Lu]Lu-PSMA-617 (PLUVICTO®, 15 Novartis). The approval of PLUVICTO® in March 2022 for patients with metastatic castration-resistant prostate cancer who have undergone prior treatments, including androgen receptor pathway-targeting agents and taxane-based chemotherapy, represents a significant advancement. Other radionuclides like 161Tb, 149Tb, 225Ac, 227Th, 223Ra, 211At, 213 Bi, 212Pb, 89Zr, and 125I are presented, emphasizing their clinical implementation or the stage of clinical trial they are in in the flow to biomedical implementation. Three clinically wise used radionuclides 177Lu, 225Ac, 223Ra are shown along with their characteristics. This review aims to elucidate the molecular mechanisms underpinning PSMA, explore the clinical applications of PSMA-targeted radiotheranostics, and critically examine the diverse challenges these therapies encounter in the treatment of prostate cancer.
Keywords: prostate cancer; prostate-specific membrane antigen; radionuclides; radiotherapy; theranostic.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Dyba T., Randi G., Bray F., Martos C., Giusti F., Nicholson N., Gavin A., Flego M., Neamtiu L., Dimitrova N., et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer. 2021;157:308–347. doi: 10.1016/j.ejca.2021.07.039. - DOI - PMC - PubMed
-
- The Internet Pathology Laboratory for Medical Education Male Genital Pathology. The University of Utah, Eccles Health Sciences Library. [(accessed on 13 May 2009)]. Available online: https://webpath.med.utah.edu/MALEHTML/MALEIDX.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

