In Silico Evaluation of Quinolone-Triazole and Conazole-Triazole Hybrids as Promising Antimicrobial and Anticancer Agents
- PMID: 40725000
- PMCID: PMC12294901
- DOI: 10.3390/ijms26146752
In Silico Evaluation of Quinolone-Triazole and Conazole-Triazole Hybrids as Promising Antimicrobial and Anticancer Agents
Abstract
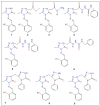
Cancer remains one of the leading causes of death globally, highlighting the urgent need for novel anticancer therapies with higher efficacy and reduced toxicity. Similarly, the rise in multidrug-resistant pathogens and emerging infectious diseases underscores the critical demand for new antimicrobial agents that target resistant infections through unique mechanisms. This study used computational approaches to investigate twenty quinolone-triazole and conazole-triazole hybrid derivatives as antimicrobial and anticancer agents (1-20) with nine reference drugs. By studying their interactions with 6 bacterial DNA gyrase and 10 cancer-inducing target proteins (E. faecalis, M. tuberculosis, S. aureus, E. coli, M. smegmatis, P. aeruginosa and EGFR, MPO, VEGFR, CDK6, MMP1, Bcl-2, LSD1, HDAC6, Aromatase, ALOX15) and comparing them with established drugs such as ampicillin, cefatrizine, fluconazole, gemcitabine, itraconazole, ribavirin, rufinamide, streptomycin, and tazobactam, compounds 15 and 16 emerged as noteworthy antimicrobial and anticancer agents, respectively. In molecular dynamics simulations, compounds 15 and 16 had the strongest binding at -10.6 kcal mol-1 and -12.0 kcal mol-1 with the crucial 5CDQ and 2Z3Y proteins, respectively, exceeded drug-likeness criteria, and displayed extraordinary stability within the enzyme's pocket over varied temperatures (300-320 K). In addition, we used density functional theory (DFT) to calculate dipole moments and molecular orbital characteristics and analyze the thermodynamic stability of putative antimicrobial and anticancer derivatives. This finding reveals a well-defined, possibly therapeutic relationship, supported by theoretical and future in vitro and in vivo studies. Compounds 15 and 16, thus, emerged as intriguing contenders in the fight against infectious diseases and cancer.
Keywords: anticancer; antimicrobial; conazole; quinolone; triazole.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Nesaragi A.R., Kamble R.R., Bayannavar P.K., Shaikh S.K.J., Hoolageri S.R., Kodasi B., Joshi S.D., Kumbar V.M. Microwave assisted regioselective synthesis of quinoline appended triazoles as potent anti-tubercular and antifungal agents via copper catalyzed cycloaddition. Bioorgan. Med. Chem. Lett. 2021;41:127984. doi: 10.1016/j.bmcl.2021.127984. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

