Circulating Extracellular Vesicles in Cardiovascular Disease
- PMID: 40725065
- PMCID: PMC12295213
- DOI: 10.3390/ijms26146817
Circulating Extracellular Vesicles in Cardiovascular Disease
Abstract
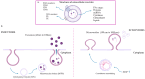
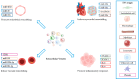
Despite notable advancements in clinical care, cardiovascular disease (CVD) remains a leading global cause of mortality. Encompassing a wide range of heart and blood vessel disorders, CVD requires targeted prevention and treatment strategies to mitigate its public health impact. In recent years, extracellular vesicles (EVs) have emerged as crucial mediators of intercellular communication, influencing key processes such as vascular remodeling, inflammation, and immune responses in CVDs. EVs, including exosomes and microvesicles, carry bioactive molecules such as miRNAs, proteins, and lipids that contribute to disease progression. They are released by various cell types, including platelets, erythrocytes, leukocytes, endothelial cells, and cardiomyocytes, each playing distinct roles in cardiovascular homeostasis and pathology. Given their presence in circulating blood and other body fluids, EVs are increasingly recognized as promising non-invasive biomarkers for CVD diagnosis and prognosis. Furthermore, EV-based therapeutic strategies, including engineered EVs for targeted drug delivery, are being explored for treating atherosclerosis, myocardial infarction, heart failure, and hypertension. However, challenges remain regarding the standardization of EV isolation and characterization techniques, which are critical for their clinical implementation. This review highlights the diverse roles of EVs in CVD pathophysiology, their potential as diagnostic and prognostic biomarkers, and emerging therapeutic applications, clearing the way for their integration into cardiovascular precision medicine.
Keywords: atherosclerosis; biomarkers; cardiovascular disease; exosomes; extracellular vesicles; heart failure; hypertension; miRNA; microparticles; myocardial infarction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Palena L.M., Isernia G., Parlani G., Veroux P., Ficarelli I., Frascheri A., Pischedda A., Patrone L., Dionisi C.P., Cianni R., et al. A multicenter prospective observational study appraising the effectiveness of the Supera stent after subintimal recanalization of femoro-popliteal artery occlusion: The SUPERSUB II study. Catheter. Cardiovasc. Interv. 2024;103:963–971. doi: 10.1002/ccd.31028. - DOI - PubMed
-
- Ekanem E., Neuzil P., Reichlin T., Kautzner J., van der Voort P., Jais P., Chierchia G.B., Bulava A., Blaauw Y., Skala T., et al. Safety of pulsed field ablation in more than 17,000 patients with atrial fibrillation in the MANIFEST-17K study. Nat. Med. 2024;30:2020–2029. doi: 10.1038/s41591-024-03114-3. - DOI - PMC - PubMed
-
- Mantovani L., Mikus E., Tenti E., Sangiorgi D., Zannoni S., Cavallucci A., Ferroni L., Cimaglia P., Tolio V., Tremoli E., et al. Post-Operative Delirium and Cognitive Dysfunction in Aged Patients Undergoing Cardiac Surgery: A Randomized Comparison between Two Blood Oxygenators. Bioengineering. 2023;10:1429. doi: 10.3390/bioengineering10121429. - DOI - PMC - PubMed
-
- Joseph J.J., Deedwania P., Acharya T., Aguilar D., Bhatt D.L., Chyun D.A., Di Palo K.E., Golden S.H., Sperling L.S., American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health et al. Comprehensive Management of Cardiovascular Risk Factors for Adults With Type 2 Diabetes: A Scientific Statement From the American Heart Association. Circulation. 2022;145:e722–e759. doi: 10.1161/CIR.0000000000001040. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

