H2O2 Sensitivity of Kv Channels in Hypoxic Pulmonary Vasoconstriction: Experimental Conditions Matter
- PMID: 40725104
- PMCID: PMC12295352
- DOI: 10.3390/ijms26146857
H2O2 Sensitivity of Kv Channels in Hypoxic Pulmonary Vasoconstriction: Experimental Conditions Matter
Abstract
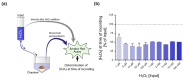
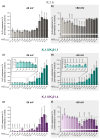
Hypoxic pulmonary vasoconstriction (HPV) optimizes gas exchange but, when impaired, can result in life-threatening hypoxemia. Moreover, under conditions of generalized alveolar hypoxia, HPV can result in pulmonary hypertension. Voltage-gated K+ channels (Kv channels) are key to HPV: a change in the intracellular hydrogen peroxide (H2O2) levels during acute hypoxia is assumed to modulate these channels' activity to trigger HPV. However, there are longstanding conflicting findings on whether H2O2 inhibits or activates Kv channels. Therefore, we hypothesized that H2O2 affects Kv channels depending on the experimental conditions, i.e., the H2O2 concentration, the channel's subunit configuration or the experimental clamping potential in electrophysiological recordings. Therefore, cRNAs encoding the Kv1.5 channel and the auxiliary Kvβ subunits (Kvβ1.1, Kvβ1.4) were generated via in vitro transcription before being injected into Xenopus laevis oocytes for heterologous expression. The K+ currents of homomeric (Kv1.5) or heteromeric (Kv1.5/Kvβ1.1 or Kv1.5/Kvβ1.4) channels were assessed by two-electrode voltage clamp. The response of the Kv channels to H2O2 was markedly dependent on (a) the clamping potential, (b) the H2O2 concentration, and (c) the Kv channel's subunit composition. In conclusion, our data highlight the importance of the choice of experimental conditions when assessing the H2O2 sensitivity of Kv channels in the context of HPV, thus providing an explanation for the long-lasting controversial findings reported in the literature.
Keywords: Kv channels; Kvβ subunits; Xenopus laevis oocytes; hydrogen peroxide (H2O2); hypoxic pulmonary vasoconstriction (HPV); two-electrode voltage clamp (TEVC).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells.Circ Res. 2004 Aug 6;95(3):308-18. doi: 10.1161/01.RES.0000137173.42723.fb. Epub 2004 Jun 24. Circ Res. 2004. PMID: 15217912
-
The Conopeptide αD-FrXXA, an Inhibitor of Voltage-Gated Potassium Channels.Mar Drugs. 2025 May 30;23(6):237. doi: 10.3390/md23060237. Mar Drugs. 2025. PMID: 40559646 Free PMC article.
-
KChAP/Kvbeta1.2 interactions and their effects on cardiac Kv channel expression.Am J Physiol Cell Physiol. 2001 Jul;281(1):C290-9. doi: 10.1152/ajpcell.2001.281.1.C290. Am J Physiol Cell Physiol. 2001. PMID: 11401852
-
The role of k+ channels in determining pulmonary vascular tone, oxygen sensing, cell proliferation, and apoptosis: implications in hypoxic pulmonary vasoconstriction and pulmonary arterial hypertension.Microcirculation. 2006 Dec;13(8):615-32. doi: 10.1080/10739680600930222. Microcirculation. 2006. PMID: 17085423 Review.
-
Education support services for improving school engagement and academic performance of children and adolescents with a chronic health condition.Cochrane Database Syst Rev. 2023 Feb 8;2(2):CD011538. doi: 10.1002/14651858.CD011538.pub2. Cochrane Database Syst Rev. 2023. PMID: 36752365 Free PMC article.
References
-
- Sommer N., Hüttemann M., Pak O., Scheibe S., Knoepp F., Sinkler C., Malczyk M., Gierhardt M., Esfandiary A., Kraut S., et al. Mitochondrial Complex IV Subunit 4 Isoform 2 Is Essential for Acute Pulmonary Oxygen Sensing. Circ. Res. 2017;121:424–438. doi: 10.1161/CIRCRESAHA.116.310482. - DOI - PMC - PubMed
-
- Lang M., Som A., Mendoza D.P., Flores E.J., Reid N., Carey D., Li M.D., Witkin A., Rodriguez-Lopez J.M., Shepard J.-A.O., et al. Hypoxaemia related to COVID-19: Vascular and perfusion abnormalities on dual-energy CT. Lancet Infect. Dis. 2020;20:1365–1366. doi: 10.1016/S1473-3099(20)30367-4. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

