Combining CSF and Serum Biomarkers to Differentiate Mechanisms of Disability Worsening in Multiple Sclerosis
- PMID: 40725145
- PMCID: PMC12295370
- DOI: 10.3390/ijms26146898
Combining CSF and Serum Biomarkers to Differentiate Mechanisms of Disability Worsening in Multiple Sclerosis
Abstract
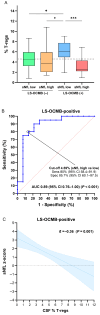
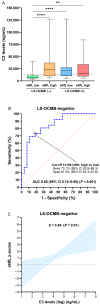
The combined use of serum and CSF biomarkers for prognostic stratification in multiple sclerosis (MS) remains underexplored. This multicenter observational study investigated associations between serum neurofilament light chain (sNfL), glial fibrillary acidic protein (sGFAP), and CSF lipid-specific IgM oligoclonal bands (LS-OCMB) with different forms of disability worsening, such as relapse-associated worsening (RAW), active progression independent of relapse activity (aPIRA), and non-active PIRA (naPIRA). A total of 535 patients with MS were included, all sampled within one year of disease onset. Biomarkers were quantified using single-molecule array and immunoblotting techniques, and CSF cell subsets were analyzed by flow cytometry. High sNfL z-scores and LS-OCMB positivity were independently associated with increased risk of RAW and aPIRA, collectively termed inflammatory-associated worsening (IAW), while elevated sGFAP levels predicted naPIRA. Patients with both high sNfL and LS-OCMB positivity had the highest risk of IAW. Among LS-OCMB-positive patients, higher regulatory T cell percentages were associated with lower sNfL levels, suggesting a protective role. Conversely, in LS-OCMB-negative patients, sNfL levels correlated with CSF C3 concentrations. These findings support the complementary role of sNfL, sGFAP, and LS-OCMB in identifying distinct mechanisms of disease worsening and may inform early personalized management strategies in MS.
Keywords: glial fibrillary acidic protein; intrathecal IgM synthesis; multiple sclerosis; neurofilament light chain; progression.
Conflict of interest statement
Monreal, E. has received speaking honoraria and travel expenses for participation in scientific meetings and has served as a steering committee member or participated in advisory boards of clinical trials in the past 3 years with Biogen, Merck, Novartis, Roche, Almirall, Johnson & Johnson, Bristol-Myers Squibb, Sanofi, and Neuraxpharma and has also acted as a scientific advisor for the CRO PSI. Sainz de la Maza, S. reported receiving personal fees from Almirall, Bristol Myers Squibb, and Teva outside the submitted work and receiving compensation for lectures or travel expenses from Merck Serono, Biogen, Sanofi Genzyme, Roche, Janssen, and Novartis. Llufriu, R. received compensation for consulting services and speaker honoraria from Biogen Idec, Novartis, TEVA, Genzyme, Sanofi-Genzyme, and Merck. Ramió-Torrentà, L. has received compensation for consulting services and speaking honoraria from Biogen, Novartis, Bayer, Merck, Sanofi, Genzyme, Janssen, Horizon, Teva Pharmaceutical Industries Ltd., Almirall, and Mylan. Martínez-Ginés, M-G. received compensation for consulting services and speaking fees from Merck, Biogen, Novartis, Sanofi-Genzyme, Almirall, BMS, Janssen, Roche, Horizon, and Viatris. Aladro, Y. has received research grants, travel support, and lecturing and consulting fees from Bayer, Biogen, Roche, Merck, Novartis, Almirall, Sanofi-Genzime, Janssen, and Bristol Myers Squibb. Cuello, J.P. received honorarium for participation in advisory boards and scientific communications, as a consultant, and for research support from Novartis, Biogen, Sanofi, and Roche. Pilo de la Fuente, B. has received travel support, lecturing fees, and teaching courses from Almirall, Merck, Novartis, Sandoz, and Sanofi-Genzime. Quiroga-Varela, A. has received support for attending congresses from Merck and Novartis. Rodríguez-Jorge, F. received speaker honoraria from Bioden Idec and Sanofi. Chico-García, J.L. received speaker fees and travel support and/or has served on advisory boards by Biogen, Sanofi, Bayer, Janssen, BMS, and Bial. Montalban, X. has received speaking honoraria and travel expenses for participation in scientific meetings and has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past 3 years with Actelion, Alexion, Bayer, Biogen, Bristol-Myers Squibb/Celgene, EMD Serono, EXCEMED, Genzyme, Hoffmann-La Roche, Immunic, Janssen Pharmaceuticals, MedDay, Merck, Mylan, MSIF, Nervgen, NMSS, Novartis, Roche, Sanofi-Genzyme, Teva Pharmaceuticals, and TG Therapeutics. Costa-Frossard, L. received speaker fees and travel support and/or has served on advisory boards by Biogen, Sanofi, Merck, Bayer, Novartis, Roche, Teva, Celgene, Ipsen, Biopas, and Almirall. Villar, L.M. received research grants, travel support, or honoraria for speaking engagements from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, Celgene, and Bristol-Myers Squib. No other disclosures are reported.
Figures

References
-
- Di Filippo M., Gaetani L., Centonze D., Hegen H., Kuhle J., Teunissen C.E., Tintoré M., Villar L.M., Willemse E.A., Zetterberg H., et al. Fluid biomarkers in multiple sclerosis: From current to future applications. Lancet Reg. Health-Eur. 2024;44:101009. doi: 10.1016/j.lanepe.2024.101009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

