Toward Safer Biotherapeutics: Expression and Characterization of a Humanized Chimeric L-Asparaginase in E. coli
- PMID: 40725166
- PMCID: PMC12295240
- DOI: 10.3390/ijms26146919
Toward Safer Biotherapeutics: Expression and Characterization of a Humanized Chimeric L-Asparaginase in E. coli
Abstract
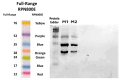
Acute lymphoblastic leukemia (ALL) is the most common cancer affecting children, making up about 80% of all acute leukemia cases in the pediatric population. While treatment with L-asparaginase (ASNase) has greatly improved survival rates, its bacterial origin often causes immune reactions in some patients, which can reduce how well the therapy works. To overcome this challenge, previous in silico studies designed a humanized chimeric ASNase by swapping out the predicted immunogenic parts of the bacterial enzyme with similar, less immunogenic segments from the human version-while keeping the enzyme's active site intact. In this study, the chimeric L-asparaginase designed was successfully cloned, expressed, and purified using the Escherichia coli Rosetta strain. The production conditions (37 °C, 0.01 mM IPTG, 2-4 h) were optimized, and we purified the enzyme in a single step with nickel-affinity chromatography. The enzyme's activity was confirmed in vitro, showing that it is possible to produce a functional humanized variant in a bacterial system. These results lay important groundwork for future research to assess the immune response and therapeutic potential of this novel chimeric enzyme.
Keywords: L-asparaginase (ASNase); acute lymphoblastic leukemia (ALL); chimeric; recombinant protein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- SEPEAP Consejo Editorial Subdirectores Ejecutivos Pediatría Integral. 2016. [(accessed on 3 June 2025)]. Available online: www.sepeap.org.
-
- Watanabe A., Miyake K., Nordlund J., Syvänen A.-C., van der Weyden L., Honda H., Yamasaki N., Nagamachi A., Inaba T., Ikawa T., et al. Association of aberrant ASNS imprinting with asparaginase sensitivity and chromosomal abnormality in childhood BCP-ALL. Blood. 2020;136:2319–2333. doi: 10.1182/blood.2019004090. - DOI - PMC - PubMed
-
- Baruchel A., Brown P., Rizzari C., Silverman L., van der Sluis I., Wolthers B.O., Schmiegelow K. Increasing completion of asparaginase treatment in childhood acute lymphoblastic leukaemia (ALL): Summary of an expert panel discussion. ESMO Open. 2020;5:e000977. doi: 10.1136/esmoopen-2020-000977. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

