Could Skin Autofluorescence Be a Useful Biomarker in Systemic Lupus Erythematosus? A Systematic Review
- PMID: 40725181
- PMCID: PMC12295628
- DOI: 10.3390/ijms26146934
Could Skin Autofluorescence Be a Useful Biomarker in Systemic Lupus Erythematosus? A Systematic Review
Abstract
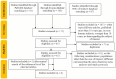
Systemic lupus erythematosus (SLE) is a multifaceted autoimmune disease with a heterogeneous organ involvement, for which reliable biomarkers are still being studied. The implication of advanced glycation end products (AGEs), resulting from oxidative stress, and their interaction with the receptor for AGEs (RAGE) has been studied in pathologies with chronic proinflammatory status, offering potential relevance in SLE. This systematic review aimed to evaluate the utility of skin autofluorescence (SAF)-a non-invasive proxy for AGE accumulation-as a biomarker for disease severity, activity, and impact in SLE patients. Following PRISMA guidelines, six studies assessing SAF and/or circulating AGEs and soluble RAGE (sRAGE) in SLE were analyzed. Findings consistently showed higher AGE levels in SLE patients compared to healthy controls, with several correlations between SAF/AGEs and disease features such as SLEDAI scores, organ involvement, inflammatory markers, and damage indices. Decreased sRAGE levels were also observed, possibly due to consumption by AGEs. Some studies further reported predictive associations between specific AGEs or their ratios with sRAGE and particular clinical phenotypes. Although heterogeneity among studies limits definitive conclusions, the AGEs-sRAGE axis-and especially SAF-emerges as a promising candidate for future biomarker development in SLE. Further large-scale longitudinal studies are needed to confirm its clinical utility.
Keywords: advanced glycation end products; biomarker; skin autofluorescence; systemic lupus erythematosus.
Conflict of interest statement
The authors declare no conflicts of interest.
Similar articles
-
Skin autofluorescence is associated with glycemic variability in type 2 diabetes patients.Sci Rep. 2025 Jul 6;15(1):24105. doi: 10.1038/s41598-025-09517-7. Sci Rep. 2025. PMID: 40619507 Free PMC article.
-
The role of advanced glycation end products (AGEs) and the receptor for AGEs (RAGE) in hypertrophic obstructive cardiomyopathy.PLoS One. 2025 Jul 24;20(7):e0328032. doi: 10.1371/journal.pone.0328032. eCollection 2025. PLoS One. 2025. PMID: 40705819 Free PMC article.
-
Skin Autofluorescence as a Potential Adjunctive Marker for Cardiovascular Risk Assessment in Type 2 Diabetes: A Systematic Review.Int J Mol Sci. 2024 Mar 31;25(7):3889. doi: 10.3390/ijms25073889. Int J Mol Sci. 2024. PMID: 38612699 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
-
- Constantinescu A., Cobilinschi C., Gradinaru E., Saulescu I., Ionescu R. Features of late-onset systemic lupus erythematosus. Rom. J. Rheumatol. 2021;30:121–124. doi: 10.37897/RJR.2021.3.6. - DOI
-
- Smolen J.S., Landewé R.B.M., Bergstra S.A., Kerschbaumer A., Sepriano A., Aletaha D., Caporali R., Edwards C.J., Hyrich K.L., Pope J.E., et al. EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2022 Update. Ann. Rheum. Dis. 2022;82:3–18. doi: 10.1136/ard-2022-223356. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical