Lipid Accumulation and Insulin Resistance: Bridging Metabolic Dysfunction-Associated Fatty Liver Disease and Chronic Kidney Disease
- PMID: 40725208
- PMCID: PMC12295539
- DOI: 10.3390/ijms26146962
Lipid Accumulation and Insulin Resistance: Bridging Metabolic Dysfunction-Associated Fatty Liver Disease and Chronic Kidney Disease
Abstract
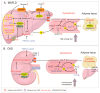
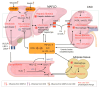
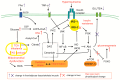
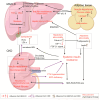
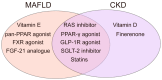
Metabolic dysfunction-associated fatty liver disease (MAFLD), a recently proposed term to replace non-alcoholic fatty liver disease (NAFLD), emphasizes the critical role of metabolic dysfunction and applies broader diagnostic criteria. Diagnosis of MAFLD requires evidence of hepatic steatosis combined with obesity, type 2 diabetes mellitus, or other metabolic dysregulation conditions, all of which significantly elevate the risk of chronic kidney disease (CKD). This review discusses the pathological mechanisms of lipid accumulation and insulin resistance in MAFLD and CKD, highlighting their mechanistic connections. Specifically, ectopic fat accumulation triggered by metabolic reprogramming, oxidative stress and inflammation induced by energy overload, modified lipids, uremic toxins, and senescence, as well as insulin resistance pathways activated by pro-inflammatory factors and lipotoxic products, collectively exacerbate simultaneous hepatic and renal injury. Moreover, interactions among hyperinsulinemia, the sympathetic nervous system, the renin-angiotensin system (RAS), and altered adipokine and hepatokine profiles further amplify insulin resistance, ectopic lipid deposition, and systemic damage. Finally, the review explores potential therapeutic strategies targeting lipid metabolism, insulin sensitivity, and RAS activity, which offer promise for dual-organ protection and improved outcomes in both hepatic and renal systems.
Keywords: chronic kidney disease; insulin resistance; lipid; metabolic dysfunction-associated fatty liver disease; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Overexpression of (P)RR in SHR and Renin-Induced HepG2 Cells Leads to Spontaneous Hypertension Combined with Metabolic Dysfunction-Associated Fatty Liver Disease.Int J Mol Sci. 2025 Jul 7;26(13):6541. doi: 10.3390/ijms26136541. Int J Mol Sci. 2025. PMID: 40650317 Free PMC article.
-
Phytotherapy as Multi-Hit Therapy to Confront the Multiple Pathophysiology in Non-Alcoholic Fatty Liver Disease: A Systematic Review of Experimental Interventions.Medicina (Kaunas). 2021 Aug 14;57(8):822. doi: 10.3390/medicina57080822. Medicina (Kaunas). 2021. PMID: 34441028 Free PMC article.
-
Metabolic dysfunction-associated steatotic liver disease: A story of muscle and mass.World J Gastroenterol. 2025 May 28;31(20):105346. doi: 10.3748/wjg.v31.i20.105346. World J Gastroenterol. 2025. PMID: 40495947 Free PMC article.
-
Alcohol Consumption and Liver Metabolism in the Era of MASLD: Integrating Nutritional and Pathophysiological Insights.Nutrients. 2025 Jul 5;17(13):2229. doi: 10.3390/nu17132229. Nutrients. 2025. PMID: 40647333 Free PMC article. Review.
-
Adipose tissue homeostasis orchestrates the oxidative, energetic, metabolic and endocrine disruption induced by binge drinking in adolescent rats.J Physiol. 2023 Dec;601(24):5617-5633. doi: 10.1113/JP285362. Epub 2023 Nov 22. J Physiol. 2023. PMID: 37994192
References
-
- Vuppalanchi R., Cruz M.M., Momin T., Shaikh F., Swint K., Patel H., Parmar D. Pharmacokinetic, Safety, and Pharmacodynamic Profiles of Saroglitazar Magnesium in Cholestatic Cirrhosis with Hepatic Impairment and Participants with Renal Impairment. Clin. Pharmacol. Ther. 2025;117:240–249. doi: 10.1002/cpt.3450. - DOI - PMC - PubMed
-
- Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., Zelber-Sagi S., Wong V.W.-S., Dufour J.-F., Schattenberg J.M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

