Targeting Bcl-xL with Navitoclax Effectively Eliminates Senescent Tumor Cells That Appear Following CEP-1347-Induced Differentiation of Glioma Stem Cells
- PMID: 40725230
- PMCID: PMC12294909
- DOI: 10.3390/ijms26146984
Targeting Bcl-xL with Navitoclax Effectively Eliminates Senescent Tumor Cells That Appear Following CEP-1347-Induced Differentiation of Glioma Stem Cells
Abstract
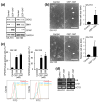
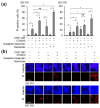
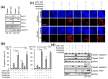
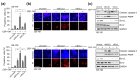
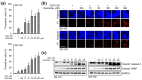
Cellular senescence is a state of the durable cell cycle arrest of dysfunctional cells, which has been associated with the promotion of tumor cell reprogramming into a stem cell state. We previously reported that the mixed lineage kinase (MLK) inhibitor CEP-1347 promotes the differentiation of glioma stem cells (GSCs)-key contributors to glioblastoma recurrence and therapy resistance-into non-stem tumor cells. However, we also noted that CEP-1347-treated GSCs exhibited a morphological change suggestive of senescence. Therefore, we herein investigated whether CEP-1347 induces senescence in GSCs and, consequently, if senescent GSCs may be eliminated using senolytics. Cell death induced by CEP-1347 in combination with senolytic agents or with the knockdown of anti-apoptotic BCL2 family genes, as well as the effects of CEP-1347 on the expression of senescence markers and anti-apoptotic Bcl-2 family proteins, were examined. The results obtained showed that CEP-1347 induced senescence in GSCs accompanied by the increased expression of Bcl-xL. Among the panel of senolytic agents tested, navitoclax, a BH3 mimetic, efficiently induced cell death in GSCs when combined with CEP-1347 at concentrations clinically achievable in the brain. The knockdown of Bcl-xL resulted in more pronounced GSC death in combination with CEP-1347 than that of Bcl-2. These results suggest that combining CEP-1347 with the targeting of Bcl-xL, the expression of which increases with CEP-1347-induced senescence, is a rational approach to ensure the elimination of GSCs, thereby improving the outcomes of glioblastoma treatment.
Keywords: drug repositioning; glioblastoma multiforme; stem cell capacity; tumor-initiating cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Senolytic-Mediated Elimination of Head and Neck Tumor Cells Induced Into Senescence by Cisplatin.Mol Pharmacol. 2022 Mar;101(3):168-180. doi: 10.1124/molpharm.121.000354. Epub 2021 Dec 14. Mol Pharmacol. 2022. PMID: 34907000 Free PMC article.
-
Apoptotic priming in senescence predicts specific senolysis by quantitative analysis of mitochondrial dependencies.Cell Death Differ. 2025 May;32(5):802-817. doi: 10.1038/s41418-024-01431-1. Epub 2025 Jan 6. Cell Death Differ. 2025. PMID: 39762561
-
Effective Targeting of Glioma Stem Cells by BSJ-04-122, a Novel Covalent MKK4/7 Dual Inhibitor.Anticancer Res. 2025 Jul;45(7):2917-2924. doi: 10.21873/anticanres.17659. Anticancer Res. 2025. PMID: 40578938
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
References
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

