Aminopeptidase A Effect on Angiotensin Peptides and Their Blood Pressure Action
- PMID: 40725237
- PMCID: PMC12296148
- DOI: 10.3390/ijms26146990
Aminopeptidase A Effect on Angiotensin Peptides and Their Blood Pressure Action
Abstract
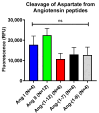
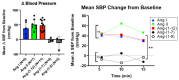
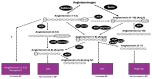
Aminopeptidase A (APA) cleaves a single aspartate residue from the amino terminus of peptides within the renin angiotensin system (RAS). Since several RAS peptides contain an N-terminal aspartate, we developed an assay to evaluate the effect of recombinant APA on the cleavage of Ang I, Ang II, Ang-(1-7), Ang-(1-9), and Ang-(1-12). The latter peptide has been proposed to be a functional Ang II-forming substrate with a hypertensive action attributable to the formed Ang II acting on AT1 receptors. Here we investigated the following: (a) the hydrolytic action of APA on Ang-(1-12), Ang I (1-10), Ang-(1-9), Ang II and Ang-(1-7) and (b) whether Ang-(1-12) pressor activity is altered by recombinant APA (r-APA) or genetic APA deficiency. We found that (a) r-APA cleaves the N-terminal aspartate of not only Ang II but also [Ang-(1-12), Ang I (1-10), Ang-(1-9)] and [Ang-(1-7)]; (b) the pressor activity of Ang-(1-12) was abolished in the presence of Lisinopril or Telmisartan; (c) r-APA significantly attenuated the pressor activities of infused Ang I and Ang II but not Ang-(1-12); and (d) r-ACE2 also did not attenuate the pressor effect of infused Ang-(1-12). Thus, in addition to increasing blood pressure indirectly via the formation of Ang II, Ang-(1-12) increases blood pressure by an Ang II-independent mechanism. We conclude that APA has an antihypertensive effect attributable to rapid degradation of Ang II, and this action may have a therapeutic potential in forms of hypertension that are Ang II-dependent. In addition, APA metabolizes Ang-(1-12), a peptide that has a prohypertensive action, in part, as a source of Ang II formation but also by a yet to be determined action independent of Ang II.
Keywords: aminopeptidase A; angiotensin; angiotensin II; angiotensin-(1-12); renin angiotensin system.
Conflict of interest statement
D.B. and J.W. are coinventors of patents entitled “Active Low Molecular Weight Variants of Angiotensin Converting Enzyme 2 (ACE2)” and “Soluble ACE2 Variants and Uses Therefor.” D.B. is the founder of Angiotensin Therapeutics Inc. D.B. has received consulting fees from Advicenne unrelated to this work and received unrelated research support from a grant from AstraZeneca. J.W. reports in the scientific advisor capacity for Angiotensin Therapeutics Inc. None of the other authors has any conflicts of interest, financial or otherwise, to disclose. The authors affirm that the study design, data acquisition, analysis, and interpretation were conducted independently of any commercial or financial interests related to the disclosed affiliations.
Figures





Similar articles
-
Increased formation of angiotensin II from angiotensin I in individuals of African descent.J Hypertens. 2025 Aug 1;43(8):1436-1441. doi: 10.1097/HJH.0000000000004068. Epub 2025 Jun 5. J Hypertens. 2025. PMID: 40509687 Clinical Trial.
-
Unlocking the potential of the ACE2/Ang-(1-7)/Mas Axis in liver diseases: From molecular mechanisms to translational applications.Diabetes Obes Metab. 2025 Aug;27(8):4069-4082. doi: 10.1111/dom.16435. Epub 2025 May 9. Diabetes Obes Metab. 2025. PMID: 40344459 Review.
-
Restoring lung renin-angiotensin system balance through blood pressure control.Clin Sci (Lond). 2025 Feb 4;139(3):215-27. doi: 10.1042/CS20241155. Clin Sci (Lond). 2025. PMID: 39905743 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Renin-angiotensin system-mediated nitric oxide signaling in podocytes.Am J Physiol Renal Physiol. 2024 Sep 1;327(3):F532-F542. doi: 10.1152/ajprenal.00316.2023. Epub 2024 Jul 18. Am J Physiol Renal Physiol. 2024. PMID: 39024356 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

