Branched-Chain Amino Acids in Parkinson's Disease: Molecular Mechanisms and Therapeutic Potential
- PMID: 40725241
- PMCID: PMC12295359
- DOI: 10.3390/ijms26146992
Branched-Chain Amino Acids in Parkinson's Disease: Molecular Mechanisms and Therapeutic Potential
Abstract
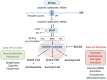
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by the selective loss of dopaminergic neurons in the substantia nigra, resulting in motor symptoms such as bradykinesia, tremor, rigidity, and postural instability, as well as a wide variety of non-motor manifestations. Branched-chain amino acids (BCAAs)-leucine, isoleucine, and valine-are essential nutrients involved in neurotransmitter synthesis, energy metabolism, and cellular signaling. Emerging evidence suggests that BCAA metabolism is intricately linked to the pathophysiology of PD. Dysregulation of BCAA levels has been associated with energy metabolism, mitochondrial dysfunction, oxidative stress, neuroinflammation, and altered neurotransmission. Furthermore, the branched-chain ketoacid dehydrogenase kinase (BCKDK), a key regulator of BCAA catabolism, has been implicated in PD through its role in modulating neuronal energetics and redox homeostasis. In this review, we synthesize current molecular, genetic, microbiome, and clinical evidence on BCAA dysregulation in PD to provide an integrative perspective on the BCAA-PD axis and highlight directions for future translational research. We explored the dualistic role of BCAAs as both potential neuroprotective agents and metabolic stressors, and critically examined the therapeutic prospects and limitations of BCAA supplementation and BCKDK targeting.
Keywords: Parkinson’s disease; amino acid metabolism; branched-chain amino acids (BCAAs); branched-chain ketoacid dehydrogenase kinase (BCKDK); gut–brain axis; mitochondrial dysfunction; neuroinflammation; precision therapeutics.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures

References
-
- Su D., Cui Y., He C., Yin P., Bai R., Zhu J., Lam J.S.T., Zhang J., Yan R., Zheng X., et al. Projections for prevalence of Parkinson’s disease and its driving factors in 195 countries and territories to 2050: Modelling study of Global Burden of Disease Study 2021. BMJ. 2025;388:e080952. doi: 10.1136/bmj-2024-080952. - DOI - PMC - PubMed
-
- Nalls M.A., Blauwendraat C., Vallerga C.L., Heilbron K., Bandres-Ciga S., Chang D., Tan M., Kia D.A., Noyce A.J., Xue A., et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18:1091–1102. doi: 10.1016/S1474-4422(19)30320-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MOST 110-2811-B-038-535/National Science and Technology Council, Taiwan
- NSTC 112-2410-H-038-/National Science and Technology Council, Taiwan
- NSTC 113-2321-B-038-007/National Science and Technology Council, Taiwan
- DP2-TMU-112-N-04/Ministry of Education, Taiwan under the 871 Higher Education Sprout Project
LinkOut - more resources
Full Text Sources
Medical

