Vitamin D3 Modulates Inflammatory and Antimicrobial Responses in Oral Epithelial Cells Exposed to Periodontitis-Associated Bacteria
- PMID: 40725246
- PMCID: PMC12295687
- DOI: 10.3390/ijms26147001
Vitamin D3 Modulates Inflammatory and Antimicrobial Responses in Oral Epithelial Cells Exposed to Periodontitis-Associated Bacteria
Abstract
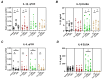
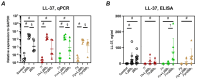
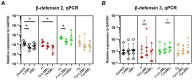
The oral epithelium is essential for maintaining oral health and plays a key role in the onset and progression of periodontitis. It serves as both a mechanical and immunological barrier and possesses antimicrobial activity. Vitamin D3, a hormone with known immunomodulatory functions, may influence oral epithelial responses. This study investigated the effects of two vitamin D3 metabolites on key immunological and antimicrobial functions of oral epithelial cells, both under basal conditions and during bacterial challenge. Ca9-22 oral epithelial cells were treated with 1,25(OH)2D3 or 25(OH)D3 in the presence or absence of Tannerella forsythia, Fusobacterium nucleatum, or Porphyromonas gingivalis. Inflammatory responses were assessed by measuring gene and protein expression of IL-1β and IL-8. Antimicrobial activity was evaluated via expression of LL-37, hBD-2, and hBD-3, as well as direct bacterial killing assays. Expression of epithelial integrity markers E-cadherin and ICAM-1 was also analyzed. Vitamin D3 metabolites reduced IL-8 expression and significantly increased LL-37 expression and production in Ca9-22 cells. Both forms enhanced antimicrobial activity against all tested pathogens and modulated epithelial integrity markers. Vitamin D3 positively regulates antimicrobial and barrier functions in oral epithelial cells, suggesting a potential role in supporting oral health and preventing periodontitis progression.
Keywords: Fusobacterium nucleatum; Porphyromonas gingivalis; Tannerella forsythia; antimicrobial activity; antimicrobial peptides; inflammation; oral epithelium; vitamin D3.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Lactobacillus lysates protect oral epithelial cells from pathogen-associated damage, increase secretion of pro-inflammatory cytokines and enhance barrier integrity.Sci Rep. 2025 Feb 18;15(1):5894. doi: 10.1038/s41598-025-86914-y. Sci Rep. 2025. PMID: 39966408 Free PMC article.
-
Antimicrobial, antibiofilm, cytotoxicity, and substantivity of aged garlic extract against oral bacteria: an in-vitro study.BMC Complement Med Ther. 2025 Jul 16;25(1):266. doi: 10.1186/s12906-025-05012-8. BMC Complement Med Ther. 2025. PMID: 40670975 Free PMC article.
-
Adjunctive antimicrobial photodynamic therapy for treating periodontal and peri-implant diseases.Cochrane Database Syst Rev. 2024 Jul 12;7(7):CD011778. doi: 10.1002/14651858.CD011778.pub2. Cochrane Database Syst Rev. 2024. PMID: 38994711 Free PMC article.
-
Peridontopathogenic key species in correlation to the current classification system.Clin Oral Investig. 2025 Jun 12;29(7):339. doi: 10.1007/s00784-025-06413-2. Clin Oral Investig. 2025. PMID: 40500387 Free PMC article.
-
Vitamin D for the management of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2024 Sep 27;9(9):CD013284. doi: 10.1002/14651858.CD013284.pub2. Cochrane Database Syst Rev. 2024. PMID: 39329240
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

