Automated Workflow for High-Throughput LC-MS/MS-Based Therapeutic Monitoring of Cannabidiol and 7-Hydroxy-cannabidiol in Patients with Epilepsy
- PMID: 40725248
- PMCID: PMC12295711
- DOI: 10.3390/ijms26146999
Automated Workflow for High-Throughput LC-MS/MS-Based Therapeutic Monitoring of Cannabidiol and 7-Hydroxy-cannabidiol in Patients with Epilepsy
Abstract
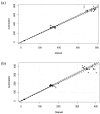
This study describes the development and validation of a fully automated workflow for serum sample preparation, enabling the quantitative determination of cannabidiol (CBD) and its active metabolite, 7-hydroxy-CBD, via liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) analysis. Implemented on an automated platform, the workflow performs key steps such as solvent dispensing, mixing, centrifugation, filtration, and supernatant transfer, producing 96-well plates ready for analysis. Human serum samples were obtained from patients with epilepsy treated with CBD. All samples were processed using both manual and automated methods to evaluate method agreement. Quantification was performed by LC-MS/MS with CBD-d3 as the internal standard (IS). Method validation was conducted in accordance with European Medicine Agency (EMA) guidelines, confirming that the automated protocol meets the recommended acceptance criteria for both intraday and interday precision and accuracy. Calibration curves demonstrated excellent linearity across the concentration ranges. Comparative analysis using Passing-Bablok regression and Bland-Altman plots demonstrated strong agreement between the methods. These findings support the clinical applicability of the automated method for the therapeutic drug monitoring (TDM) of CBD and 7-hydroxy-CBD, and its robust performance and scalability provide a solid foundation for the development of an expanded analytical panel covering a broader range of antiseizure medications (ASMs), enabling more standardized TDM protocols in clinical practice.
Keywords: 7-hydroxy-CBD; CBD; LC-MS/MS; TDM; automation; epilepsy.
Conflict of interest statement
V.D.G. has served on scientific advisory boards for Longboard Pharmaceuticals, has received research funding from Jazz Pharmaceuticals, and has received speaker and consultancy fees from Jazz Pharmaceuticals, Novartis, Nutricia, Vitaflo, and Dr. Schar Kanso, all unrelated to the present study. E.R. has received speaker honoraria, research support, and has served on the advisory board for Angelini Pharma, Arvelle Therapeutics, Eisai, Ethypharm, GW Pharmaceuticals, Jazz Pharmaceuticals, Kolfarma, Lundbeck, Pfizer, and UCB. C.T., over the past three years, has obtained funding and/or investigational product support from AbbVie and Novartis for an investigator-initiated study. She has received consultancy payments for serving on advisory boards for AbbVie, Dompé, Eli Lilly, Ipsen, Lundbeck, Medscape, Pfizer, and Teva; lecture fees and honoraria for scientific presentations from AbbVie, Eli Lilly, Lundbeck, Pfizer, and Teva; and financial assistance to attend scientific meetings from AbbVie, Eli Lilly, Dompé, Ipsen, Teva, Lundbeck, and Pfizer. Additionally, she has acted as principal investigator in clinical trials sponsored by AbbVie, Biohaven, Eli Lilly, Ipsen, Lundbeck, Pfizer, and Teva and has received grants from the European Commission, the Italian Ministry of Health, and the Italian Ministry of University. A.B. is an employee of Cytiva. T.M.C. is an employee of B.S.N. srl. The remaining authors declare no conflicts of interest.
Figures







Similar articles
-
Therapeutic Drug Monitoring of 6 New-Generation Antiseizure Medications Using a Mass Spectrometry Method: Analysis of 2-Year Experience in a Large Cohort of Korean Epilepsy Patients.Arch Pathol Lab Med. 2025 Jan 1;149(1):67-74. doi: 10.5858/arpa.2023-0386-OA. Arch Pathol Lab Med. 2025. PMID: 38576184
-
Development and validation of a high-throughput LC-MS/MS bioanalytical method for the simultaneous quantification of cannabidiol and metabolites in human plasma.J Chromatogr B Analyt Technol Biomed Life Sci. 2025 Sep 1;1263:124694. doi: 10.1016/j.jchromb.2025.124694. Epub 2025 Jun 3. J Chromatogr B Analyt Technol Biomed Life Sci. 2025. PMID: 40482367
-
Therapeutic Drug Monitoring of Everolimus Using Volumetric Absorptive Microsampling and Quantitative Dried Blood Spot Methods with LC-MS/MS in Adult Solid Organ Transplant Recipients: An Analytical and Clinical Comparative Study.Molecules. 2025 Jul 26;30(15):3139. doi: 10.3390/molecules30153139. Molecules. 2025. PMID: 40807312 Free PMC article.
-
Therapeutic monitoring of anti-seizure medications in low- and middle-income countries: a systematic review.Wellcome Open Res. 2024 Jan 31;6:92. doi: 10.12688/wellcomeopenres.16749.3. eCollection 2021. Wellcome Open Res. 2024. PMID: 37457427 Free PMC article.
-
Efficacy and safety of six new antiseizure medications for adjunctive treatment of focal epilepsy and epileptic syndrome: A systematic review and network meta-analysis.Epilepsy Behav. 2024 Mar;152:109653. doi: 10.1016/j.yebeh.2024.109653. Epub 2024 Jan 25. Epilepsy Behav. 2024. PMID: 38277848
References
-
- Li H., Liu Y., Tian D., Tian L., Ju X., Qi L., Wang Y., Liang C. Overview of cannabidiol (CBD) and its analogues: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer’s disease. Eur. J. Med. Chem. 2020;192:112163. doi: 10.1016/j.ejmech.2020.112163. - DOI - PubMed
-
- Epidyolex . Summary of Product Characteristics. GW Pharma (International) B.V.; London, UK: 2019. [(accessed on 2 April 2025)]. Available online: https://www.ema.europa.eu/en/documents/product-information/epidyolex-epa....
-
- Epidiolex, Full. Prescribing Information. Greenwich Biosciences Inc. 2020. [(accessed on 2 April 2025)]. Available online: https://www.lice.it/LICE_ita/commissione_farmaco/farmaco21/epidyolex-epa....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

