Multivalent Immune-Protective Effects of Egg Yolk Immunoglobulin Y (IgY) Derived from Live or Inactivated Shewanella xiamenensis Against Major Aquaculture Pathogens
- PMID: 40725257
- PMCID: PMC12295794
- DOI: 10.3390/ijms26147012
Multivalent Immune-Protective Effects of Egg Yolk Immunoglobulin Y (IgY) Derived from Live or Inactivated Shewanella xiamenensis Against Major Aquaculture Pathogens
Abstract
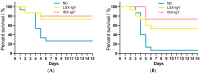
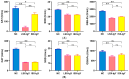
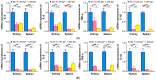
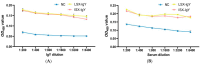
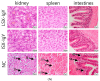
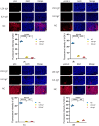
Egg yolk immunoglobulin Y (IgY) possesses advantages such as low cost, easy availability, simple preparation, high antigen specificity, absence of drug residues, and compliance with animal welfare standards, making it an environmentally friendly and safe alternative to antibiotics. This research utilizes IgY antibody technology to develop a multivalent passive immune vaccine for major pathogenic bacteria in aquaculture. In this study, IgY antibodies against live Shewanella xiamenensis (LSX-IgY) and inactivated S. xiamenensis (ISX-IgY) were prepared by immunizing laying hens, and passive immunization protection experiments were conducted in Carassius auratus infected with S. xiamenensis and Aeromonas hydrophila. The passive immunization protection rates of LSX-IgY and ISX-IgY against S. xiamenensis were 63.64% and 72.73%, respectively, and the passive cross-protection rates against A. hydrophila were 50% and 71.43%, respectively. Further, C. auratus sera could specifically bind to S. xiamenensis or A. hydrophila in vitro, and the phagocytic activity of leukocytes was increased. LSX-IgY and ISX-IgY could reduce the bacterial load in the C. auratus kidneys. Meanwhile, they could significantly reduce the levels of antioxidant factors in serum and inhibit the mRNA expression of inflammation-related factors in the kidneys and spleens. Additionally, histopathology and immunofluorescence analysis showed that both IgY preparations preserved tissue integrity and reduced the expression of apoptosis factor (p53) and DNA damage factor (γH2A.X) of visceral organs, respectively. In summary, LSX-IgY and ISX-IgY can combat various bacterial infections, with no significant difference between the two. Additionally, inactivated bacterial immunization is more aligned with animal welfare standards for laying hens. Therefore, ISX-IgY is expected to serve as a multivalent vaccine against major aquaculture pathogens.
Keywords: Aeromonas hydrophila; IgY antibody; Shewanella xiamenensis; multivalent vaccine; passive immunity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Differential polyvalent passive immune protection of egg yolk antibodies (IgY) against live and inactivated Vibrio fluvialis in fish.Fish Shellfish Immunol. 2024 Aug;151:109751. doi: 10.1016/j.fsi.2024.109751. Epub 2024 Jul 4. Fish Shellfish Immunol. 2024. PMID: 38971349
-
Decreased pathogenicity of triple-mutant of Aeromonas hydrophila flgK, flgL, flgE suggests its potential as a live attenuated vaccine for Carassius auratus.Fish Shellfish Immunol. 2025 Oct;165:110486. doi: 10.1016/j.fsi.2025.110486. Epub 2025 Jun 16. Fish Shellfish Immunol. 2025. PMID: 40516799
-
Protection of Nile tilapia against Aeromonas hydrophila using a cobalt oxide nanoparticle vaccine containing inactivated whole cell bacteria.Dev Comp Immunol. 2025 Aug;169:105409. doi: 10.1016/j.dci.2025.105409. Epub 2025 Jul 1. Dev Comp Immunol. 2025. PMID: 40609713
-
Effect of chicken egg yolk antibodies (IgY) against diarrhea in domesticated animals: a systematic review and meta-analysis.PLoS One. 2014 May 20;9(5):e97716. doi: 10.1371/journal.pone.0097716. eCollection 2014. PLoS One. 2014. PMID: 24846286 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 2022AH051330/the Key Projects of Scientific Research Plan of Colleges and Universities of Anhui Province
- GXXT-2023-077/the University Collaborative Innovation Project of Anhui Province
- Anhui Education Secretary Department [2023]13/the Biological and Medical Sciences of Applied Summit Nurturing Disciplines in Anhui Province
- S202210371092 and S202310371056/the College Student Innovation and Entrepreneurship Project of Anhui Province
- 2022AH010012/the Outstanding Innovative Research Team for Molecular Enzymology and Detection in Anhui Provincial Universities
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

