Development of a Stage- and Species-Specific RNAi System for Molecular Insights in Trichogramma Wasps
- PMID: 40725304
- PMCID: PMC12294811
- DOI: 10.3390/insects16070673
Development of a Stage- and Species-Specific RNAi System for Molecular Insights in Trichogramma Wasps
Abstract
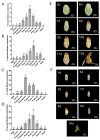
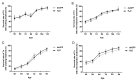
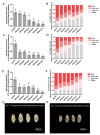
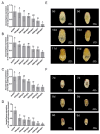
Trichogramma wasps, egg parasitoids widely used to control lepidopteran pests, have long eluded in-depth molecular mechanistic studies due to their minute size and genetic tool scarcity. While previous RNAi efforts were restricted to T. dendrolimi, we developed the first cross-species RNAi system for both T. dendrolimi and the previously intractable T. ostriniae. Temporal expression profiling identified white and laccase 2 as stage-specific RNAi targets, peaking during prepupal/pupal stages, which were tested across species and developmental stages using microinjection and soaking dsRNA delivery methods. Survival analysis prioritized soaking for T. dendrolimi prepupae/pupae, while microinjection was essential for T. ostriniae to bypass prepupal mortality during soaking. Concentration-dependent RNAi targeting the white gene achieved 85.61% transcript reduction in T. dendrolimi via soaking and 89.36% in T. ostriniae via microinjection at 2000 ng/μL, correlating with 64.06% and 32.09% white-eyed pupae, causing a significant reduction in eye pigments. For the laccase 2 gene, soaking at 2000 ng/μL induced 88.35% transcript reduction in T. dendrolimi and 73.31% in T. ostriniae, leading to incomplete cuticle tanning and sclerotization. This study resolves the long-standing challenge of genetic manipulation in Trichogramma wasps, providing a universally applicable framework to decipher parasitoid-host interactions at the molecular scale, which is useful for sustainable pest management strategies.
Keywords: RNA interference; Trichogramma wasps; microinjection; soaking; species-specific optimization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
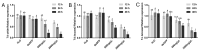
Similar articles
-
Identification of compounds from the egg volatiles of Asia corn borer, Ostrinia furnacalis, that attract egg parasitoid, Trichogramma ostriniae.Pest Manag Sci. 2025 Jul 28. doi: 10.1002/ps.70103. Online ahead of print. Pest Manag Sci. 2025. PMID: 40717536
-
Samia ricini: The promising large host eggs outclass Antheraea pernyi for rearing Trichogramma species.Insect Sci. 2025 Aug 4. doi: 10.1111/1744-7917.70145. Online ahead of print. Insect Sci. 2025. PMID: 40761002
-
Transgenerational effects of heavy metal contamination on two Trichogramma egg parasitoids and potential impacts on biological control.Insect Sci. 2025 Jul 7. doi: 10.1111/1744-7917.70125. Online ahead of print. Insect Sci. 2025. PMID: 40619954
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Cherif A., Mansour R., Grissa-Lebdi K. The egg parasitoids Trichogramma: From laboratory mass rearing to biological control of lepidopteran pests. Biocontrol. Sci. Technol. 2021;31:661–693. doi: 10.1080/09583157.2020.1871469. - DOI
-
- Lu Y., Deng X., Zhu Q., Wu D., Zhong J., Wen L., Yu X. The dsRNA Delivery, Targeting and Application in Pest Control. Agronomy. 2023;13:714. doi: 10.3390/agronomy13030714. - DOI
-
- Zhongzheng M., Cao L.-J., Chen J.-C., Chen W.-B., Shen X., Song W., Yang F., Wei S.-J. A nanocarrier-mediated dsRNA oral delivery enhances RNAi efficiency in thrips. Entomol. Gen. 2024;44:601–611.
Grants and funding
LinkOut - more resources
Full Text Sources

