Proteomic Responses of the Springtail Folsomia candida to Drought
- PMID: 40725337
- PMCID: PMC12295829
- DOI: 10.3390/insects16070707
Proteomic Responses of the Springtail Folsomia candida to Drought
Abstract
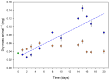
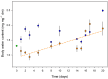
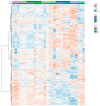
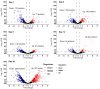
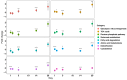
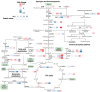
Springtails are adapted to life in the pore space of soil, where humidity in moist soil is close to saturation. Drought is the most important limiting factor for springtails; however, their molecular and physiological adaptations to low humidity are not well understood. The present study explored the global proteomic drought response of the springtail, Folsomia candida (Isotomidae, Collembola). In relatively dry soil (-360 kPa), adult springtails initially lost body water but re-established the normal body water content over the following two weeks. Nano LC-MS/MS analysis identified a total of 1729 unique proteins. Proteomic analysis and pathway enrichment found that the proteome generally did not show a dramatic induction of proteins in response to drought stress. After an initial down-regulation of pathways related to metabolism and growth, these pathways gradually returned to the same levels as in moist soil. Other pathways such as the cytoskeleton pathway, which is important in cell proliferation and differentiation, were predominantly down-regulated throughout the experiment in drought-exposed animals, which correlated with essentially no somatic growth of the springtails in dry soil. This study facilitates the understanding of the consequences of climate change on soil functioning and fertility.
Keywords: Collembola; desiccation; metabolism; pathway analysis; protein regulation.
Conflict of interest statement
Author Steffen Y. Bak was employed by the International Flavors & Fragrances Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bødvarsson H. Alimentary studies of seven common soil-inhabiting Collembola of southern Sweden. Entomol. Scand. 1970;1:74–80.
-
- Ngosong C., Raupp J., Richnow H.H., Ruess L. Tracking Collembola feeding strategies by the natural C-13 signal of fatty acids in an arable soil with different fertilizer regimes. Pedobiologia. 2011;54:225–233. doi: 10.1016/j.pedobi.2011.02.004. - DOI
-
- Potapov A.A., Semenina E.E., Korotkevich A.Y., Kuznetsova N.A., Tiunov A.V. Connecting taxonomy and ecology: Trophic niches of collembolans as related to taxonomic identity and life forms. Soil Biol. Biochem. 2016;101:20–31. doi: 10.1016/j.soilbio.2016.07.002. - DOI
-
- Bardgett R.D., Chan K.F. Experimental evidence that soil fauna enhance nutrient mineralization and plant nutrient uptake in montane grassland ecosystems. Soil Biol. Biochem. 1999;31:1007–1014.
LinkOut - more resources
Full Text Sources

